A building or house is constructed with the help of bricks, stones and other solid materials. And, the water we drink, the lake, and the sea, etc. are in the liquid form. Then, the air we breathe and the gases in our atmosphere are in a gaseous stage.
Therefore, generally, we can classify the matters into three types these are
Therefore, generally, we can classify the matters into three types these are
- Solid state
- Liquid state
- Gaseous state
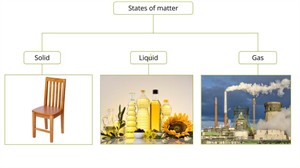
States of matter
1. Solid state:
The objects which have a definite shape, distinct boundaries and a fixed volume is called as solid objects, or they are in solid-state.
Example:
A book, pen, pencil.
A car, bike, plan, bus, etc.
All the objects which have definite shape falls into the category of solid.
The solid objects tend to stay on their original shape despite the external force.

Rocks
For example, if we apply external force to the piece of chalk, it will break into pieces, but the nature of the particles will not be altered as solids are rigid bodies.
Remember that the molecules inside the solids are so condensed, and this is the reason that they are rigid and maintain the definite shape.
Intermolecular Force on solids:
The intermolecular force in the solid is the strongest force compare to the liquid and gas. This strongest inter molecule force makes the solid matter denser and gives a certain shape and structure to it.
2. Liquid state:
In our day to day life, we come across many liquid state matters, such as the water we drink, the oil we use for cooking, the fruit juice we often drink, etc. These are all the matters in a liquid state.

Friut juices
Liquids do not have a certain shape, but it can take the shape of the container when we pour it in the container. For example, we might have seen the juice bottles. The liquid inside the bottle takes the shape of the bottle.
- An intermediate phase between the solid and gas.
- Liquids have a fixed volume but no fixed shape.
- It has a capability to take the shape of the container without changing it's volume.
Intermolecular Force on Liquid:
The intermolecular force in liquid is not high as compared to solid, but it's higher than gas.
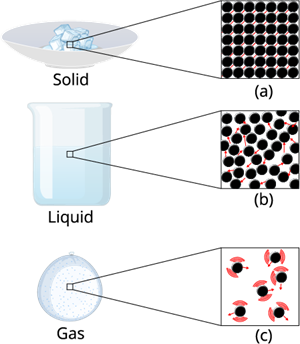
Intermolecular force of matter
3. Gaseous state:
While your mother cooks in the kitchen, you can sense the delicious aroma coming to you even though you're in another room. Have you ever wondered how that smell came to you?
And, we all like balloons, right? But, have you ever wondered how the balloon seller fill those a large number of balloons in a short span of time? The answer is gases.

Gas characteristics
Gases are highly compressible as compared to solids and liquids. In the gaseous state, the particles move about randomly at high speed.
Because of this property, we can use the gases in LPG in highly compressed condition. If we compress the gas particles, we can store it in a small container too.
We know that gas particles have more freely movable particles, so it can travel through the air easily. That's why the aroma of a food reaches to you even though you're in a separate room.
Intermolecular force on gases:
The inter molecule force in the gaseous substance is weaker as compare to the liquid and solid. This weaker inter molecule force makes the gaseous matter to move freely in the air.
Transformation of states:
Have you ever wondered why ice turns into puddles of water on a sweltering day?
Knowing that matters can change its states; when a state change occurs, the properties of substances also change. Similarly, if the state change is reversed, the substance will recover the properties which it had before.
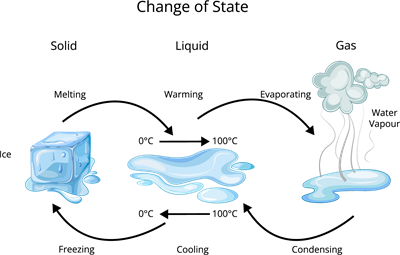
Transformation of states of matter
Let us do the following experiments to understand this concept clearly.
Activity
Experiment 1: Take some water in a vessel and heat it in medium flame. After a couple minutes observe what happens.
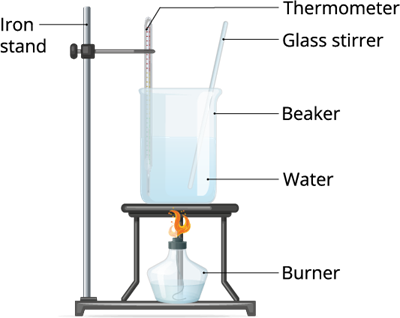
Boiling of water
Experiment 2: Take some ice pellets and keep it in room temperature or expose it in a low flame. Observe what happens to those ice pellets after a couple of minutes.
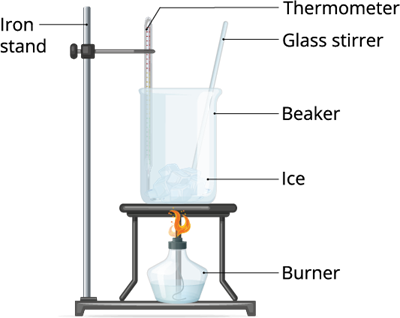
Melting of ice
Result:
In the above two experiments, we treated the liquid and the solid (ice) with heat. And, we can observe that both liquid and the solid changes its state.
On applying heat energy to the liquid, it transforms to gas. And, heating the the ice, it becomes liquid. Therefore, we can define the above process as vaporization and condensation.
Vaporization: The process where liquid converts its physical state to gaseous state is known as vaporization.
Condensation: The process of the gas converting its physical state to liquid state is known as Condensation. This is also called as reverse vaporization.
Activity 2:
Take a few camphor and place it a watch glass, and heat it. Cover the watch glass with a funnel and observe the change.
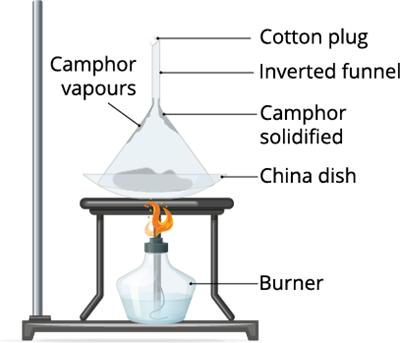
Observation: When the camphor is heated it directly changes its state from solid to gas. This process is called sublimation.
The matter changes its state respective to the energy applied to it. The following figure explains how the matter changes its state wherever the energy is applied.
Transformations of states
This leads to the rearrangement to the substance because the attracting force no longer holds the particles tightly. This same will happen if liquid heated, the attractive forces between the molecules break, and transfers to gaseous state.
Boiling Point:
The temperature at which a liquid begins to boil at the atmospheric pressure is known as its boiling point.
Similarly, when the solid objects are heated, the attractive force between the molecules breaks, then the particles leave their fixed positions and start moving more freely.
Melting Point:
The temperature at which a solid substance melts to become a liquid at the atmospheric pressure is called the melting point of that substance.