Imagine walking into a chemistry laboratory where every substance has its unique personality. A few substances combine, while a few break down, and a few kick off the other and take their place. By comparing the prior knowledge of physical and chemical changes, let us explore the different types of chemical reactions.

Exploring the chemical reactions
As we explore different chemical reactions, we come across an interesting atom - Carbon. It is an 'element of life' because the survival of living organisms depends on carbon compounds.
Learning objective:
- Recall and strengthen chemistry concepts learned in lower classes, and handle deeper concepts with confidence.
- Understanding physical and chemical changes and applying them in learnig the types of chemical reactions.
- Highlighting the importance of neutralisation reaction in daily life, and connecting it with the formation of salts and its uses.
- Applying the law of conservation of mass in balancing the chemical reactions.
- Recognising the versatility of carbon and enhancing the ability to identify and differentiate organic compounds.
1. Physical change:
Class 7 concepts:
Only change in shape, size, or state. These changes are reversible. There is no formation of a new substance.
- Freezing - heat released
- Melting - heat absorbed

Melting of chocolate; freezing of water
Class 10 concepts:
The heat changes observed during physical changes help in understanding the energy changes involved in the reactions.
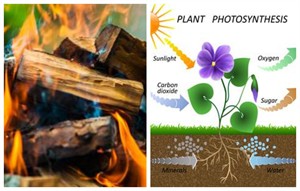
Exothermic and endothermic reaction
i. In exothermic reactions, heat is released or liberated to the surroundings.
Example: Burning of wood
ii. In endothermic reactions, heat is absorbed from the surroundings.
Example: Photosynthesis
2. Chemical change:
Class 7 concept:
- The chemical change involves the formation of a new substance. These changes are irreversible.
- Indicators: Colour change, evolution of gas, change in temperature, heat and light, and formation of precipitate.
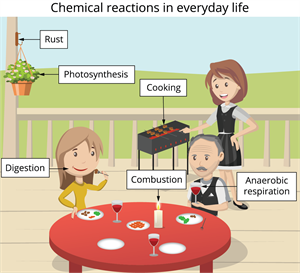
Chemical changes in everyday life
Class 10 concept:
Chemical changes such as burning, heating, photosynthesis, respiration, and rusting connects to the different types of chemical reactions.
A chemical reaction involves the breaking of existing chemical bonds and the formation of new chemical bonds.
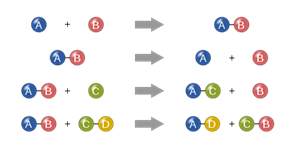
Types of chemical reactions
i. Combination reaction
ii. Decomposition reaction
iii. Displacement reaction
iv. Double displacement
Rusting of iron is an oxidation reaction, which explains the reaction of iron with air and water.

Rusting of iron
Concept interlinking: Differentiating between reaction types and the oxidation process helps understand the corrosion and rancidity.
3. Neutralisation reaction:
Class 7 concept:
- Neutralisation is the reaction between an acid and a base to form a salt and water.
\(Acid + Base \to Salt + Water\)
- The role of neutralisation reactions in everyday life, such as in solutions for heartburn, providing relief from stinging aches, aiding soil therapy, treating tooth decay, and neutralising acidic industrial wastes.
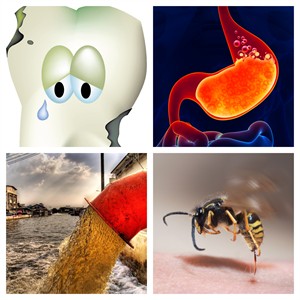
Neutralisation reactions in everyday life
Class 10 concept:
- Neutralisation reaction is a type of double displacement reaction.
- To determine the nature of the salt whether it is acidic, basic or neutral using pH.
- Formation and applications of salts such as baking soda, washing soda, and bleach.

Water of crystallisation of copper sulphate
Concept interlinking: In understanding the water of crystallisation, salts trap water molecules inside the crystal lattice during evaporation.
4. Law of conservation of mass:
Class 9 concept:
- The mass can neither be created nor be destroyed.
- \(Mass\ of\ the\ reactant = Mass\ of\ the\ product\)
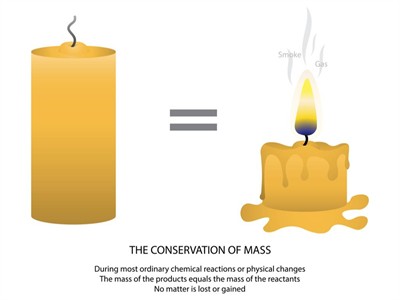
Conservation of mass
Class 10 concept: Balncing equation
- A chemical reaction is a method that involves the conversion of one or more substances (reactants) into one or more new substances (products).
- The law of conservation of mass lays the foundation in balancing the chemical reactions.
- Chemical equations are balanced because mass must remain constant.
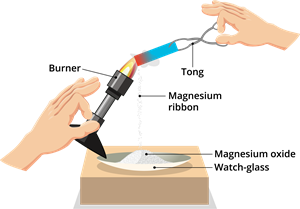
Burning of magnesium ribbon
- Unbalanced reaction: \(Mg + O_2 \to MgO\)
- Balanced reaction: \(2Mg + O_2 \to 2MgO\)
5. Formation of fuels:
Class 8 concept:
- Fossil fuels are formed due to the degradation of dead plants and animals buried under the soil, sea billions of years ago due to natural calamities.
- The degradation of organic matter occurs under extreme conditions such as the absence of air, high pressure and temperature.
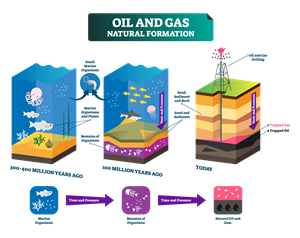
Formation of oil and gas
i. Coal - mostly carbon
ii. Natural gas - methane and other hydrocarbon
iii. Crude oil - hydrocarbon
Class 10 concept:
The formation of fossil fuels and its composition helps in understanding the physical properties of organic compounds.
- Organic compounds are mostly made of carbon and hydrogen.
- They are highly flammable.
- They have low melting and boiling point.
- Organic compounds have complex structures.
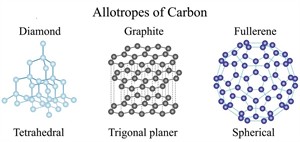
Allotropes of carbon
Concept interlinking: To understand the complex structures of organic compound and their unique characteristics such as isomerism, allotropy, and catenation.
6. Combustion and flame:
Class 8 concept:
The chemical process in which substances burn in the presence of oxygen to liberate energy in the form of heat is called combustion.
- Complete combustion: The substance burns in presence of sufficient oxygen with a blue flame and no smoke is produced.
- Incomplete combustion: The substance burns in presence of insufficient oxygen with a yellow and sooty flame.

Flame: Complete and incomplete combustion
Class 10 concept:
Hydrocarbons follow the same principles of combustion of materials. The flame colour depends on the structure of the hydrocarbon. It helps in understanding the nature of bonding in hydrocarbons.
- Saturated compounds: Burn with clean blue flame. The compound is saturated if single bonds connect all of the carbon atoms in the chain.
Example: LPG is composed saturated compounds burns with blue flame.

Combustion in LPG and kerosene stoves
- Unsaturated compounds: Burn with yellow and sooty flame. The double or triple bonds between the carbon atoms, the compound is unsaturated.
Example: Kerosene is an unsaturated compounds burns with yellow and sooty flame.
Concept interlinking: Through the nature of bonding can understand the chemical reactions of hydrocarbons such as addition, substituion, hydrogenation, and dehydrogenation.
Important!
The key ideas of atoms, compounds, and chemical changes has strengthened the base to understand the reactions, equations, and carbon compounds. Keep this readiness and curiosity alive as we are steping into the transition to Grade 10.