Chemistry becomes more exciting when ideas connect and come to life. We have already learned about atoms, molecules, and how matter is composed. As you transition into Grade 10, you will explore how atoms rearrange, how bonds break and form, and the principles that govern chemical reactions.
Mysteries in chemistry
This comparison will highlight prior knowledge from Class 8 and Class 9 and illustrate how these familiar topics evolve into more complex concepts, enabling understanding, logical reasoning, and critical thinking skills.
Learning objective:
- Recall and strengthen chemistry concepts learned in lower classes, and improve confidence.
- Understanding the structure of an atom and the nature of bonding and ionic compound formation.
- Learning to write chemical formulae using the criss-cross method and converting word equations into chemical equations.
- Applying the understanding of the natural indicators to learn about the pH and strength of acids and bases.
- Understanding the physical properties of both metals and non-metals will enhance the logical reasoning about the chemical nature of the materials.
1. Atoms:
Imagine atoms as the rooms of a house. One room is different from the other. Each and every room, the bedroom, kitchen, and living room, has its own size, design, and purpose. But together, they make a complete home. Similarly, atoms are tiny units with their own features, and when they join, they form complete substances.

Rooms of a home
Class 9 concept:
- Atoms are the smallest unit of an element involved in chemical reactions.
- Atoms are made up of sub-atomic particles.
Example: Hydrogen, chlorine, sodium
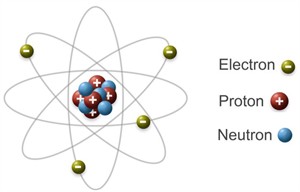
Structure of an atom
Atoms always try to achieve stability, and their goal is to attain an electron arrangement like that of the noble gases.
Class 10 concept:
-
Atoms, to attain stability by completing their octet, tend to lose or gain electrons, which leads to the formation or breaking of chemical bonds.
-
Atoms achieve the noble gas configuration by:i. Transfer of electrons - Ionic bond - (Eg: \(NaCl\))ii. Sharing of electrons - Covalent bond - (Eg: \(CCl_4\))
Concepts interlinking:
From the nature of bonding, one can distinguish the characteristics of ionic and covalent compounds, such as boiling points and melting points, and the dissociation of ions in water.
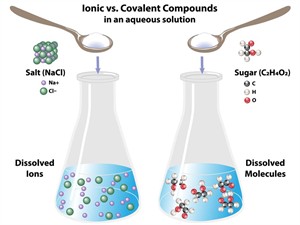
Dissociation of compounds
2. Valency and Ion formation:
Class 9 concept:
Valency:
- Atoms are more stable when they attain the octet rule ( 8 electrons in their valence shell).
- Valency is the combining capacity of an atom or ion. It is the number of electrons an atom can gain, lose, or share to achieve a stable electronic configuration.
Example: Hydrogen - \(1\); Oxygen - \(2\); Chlorine - \(1\)
Valency helps in writing chemical formulae for the compounds.
Ion formation:
An ion is formed when an atom loses or gains electrons. The electrical charge of ions is either positive or negative.
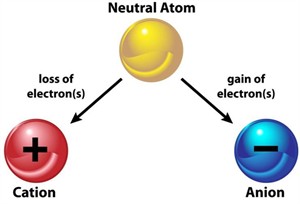
Formation of ions
- Cation - loss of electrons - \(Na \to Na^+ + e^-\)
- Anion - gain of electrons - \(Cl + e^- \to Cl^-\)
Class 10 concept: Ionic compound formation
From the concept of ion formation, understanding the formation of ionic compounds,
- Ions do not exist freely in the solid state; they combine to form a compound.
- The positive ion (cation) and the negative ion (anion) combine to form an ionic compound. The compounds are electrically neutral.
Example: The cation \(Ca^{2+}\) combine with the anion \(O^2-\) to for an ionic compound calcium oxide (\(CaO\)).

Formation of calcium oxide
Concept interlinking:
Connecting the ion formation to the properties of ionic compounds. As ionic compounds are formed from oppositely charged ions, they have,
Strong electrostatic force of attraction \(→\) hard solid \(→\) high melting and boiling point
3. Criss-cross method:
Class 9 concept:
In this method, the valencies of the combining atoms must be crossed.
i. Formula of calcium oxide:
The symbol for calcium \(Ca\) and oxygen \(O\)
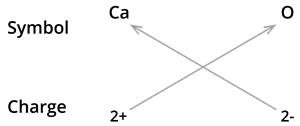
Formula: \(CaO\)
Class 10 concept:
- Writing chemical formulae for different compounds.
- Converting the word equation to a chemical equation.
Example:
\(Calcium + Oxygen \to Calcium\ oxide\)
\(2Ca + O_2 \to 2CaO\)
Concept interlinking:
Writing chemical equations promotes understanding of different types of chemical reactions.
4. Acid-base indicators:
Class 7 concept:
-
Acids taste sour, bases taste bitter.
-
Using natural indicators such as litmus paper, turmeric, red rose, and china rose to determine whether a substance is acidic or basic.
Litmus test
Class 10 concept:
Indicators help in understanding the concept of pH (potential of hydrogen):
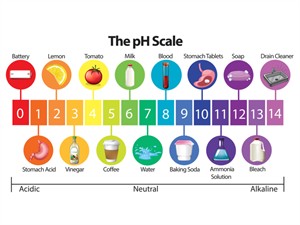
pH scale
-
Acids and bases show different colours in indicators because their \(H^+\) and \(OH^-\) ion concentrations change the colour of the indicator.
-
The pH scale (\(0 – 14\)) is used to measure acidity and basicity more precisely.
Concept interlinking:
The pH of acids and bases indicates the concentration of ions and the strength of acids and bases. To understand the properties of acids and bases.
5. Metals and non-metals:
Class 9 concept:
The physical properties of metals and non-metals.
- Metals are shiny, hard, malleable, ductile and good conductors.

Properties of metals
- Non-metals are dull, soft, brittle, and poor conductors.

Properties of non-metals
Class 10 concept:
From the physical properties connecting it with the electrical conductivity of compounds, metallurgical nature and chemical reactions of metals and non-metals.
i. Free electrons/ions - responsible for conductivity.
ii. Metallic bonding - making metals malleable and ductile.
iii. Ions \(→\) Electron transfer between metals and non-metals \(→\) Ionic compounds.
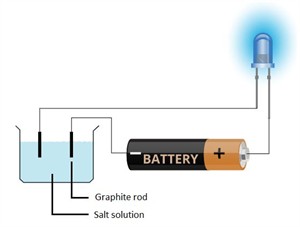
Conductivity of salt solution
Concept interlinking:
The physical nature of the metals help to understand the reactivity series, which explains the chemical reactions of metals with air, water, acids and salts.
As the familiar concepts are revisiting with deeper explanations, understanding strengthens, and the ability to reason and apply improves.
Reference:
https://shmector.com/free-vector/houses/house_with_rooms/7-0-1671