Choose the best answer:
1. Ashwin have three aqueous solutions A, B and C as given below:
A. Potassium nitrate
B. Ammonium chloride
С. Sodium carbonate
The ascending order of the pH of these solutions is:
A. Potassium nitrate
B. Ammonium chloride
С. Sodium carbonate
The ascending order of the pH of these solutions is:
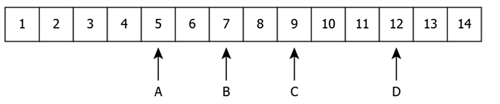
2. The following table shows the pH values of four solutions A, B, C and D on a pH scale.
The solutions A, B, C and D respectively are:
The solutions A, B, C and D respectively are: