Have you ever noticed an iron gate gradually change to a reddish-brown over time? Or seen a matchstick ignite into flames in a few seconds? Surprisingly, both are examples of chemical changes! Let’s explore two distinct types of reactions,
- Rusting – A slow and quiet change
- Combustion – A rapid and intense change

Burning of a matchstick
A flame on a candle or rust appearing on iron are both indications that matter is changing irreversibly and undergoing chemical change.
1. Rusting:
The usage of iron and steel articles has become an integral part of our daily life. People use iron for a wide range of daily life items, making bridges, ships, cars, truck bodies, grills in windows and many other articles.
Chemical reactions on the surface of shining metals and other items cause these articles to lose their shine. When exposed to atmospheric air, silver articles turn black.

Rusted iron chain
Iron material loses its shine and turns into a red-brown flaky substance when exposed to air and moisture in the atmosphere for an extended period, a process known as rusting. It is a natural process that is irreversible, resulting in the formation of a new substance.
The chemical process of rusting is as follows,
\(Iron\) \(+\) \(Oxygen\) \(+\) \(Water\) \(\to\) \(Iron\) \(oxide(rust)\)
\(Fe + O_2 + H_2O \to Fe_2O_3\)
Rusting occurs more rapidly in environments with high humidity (i.e., a high moisture content in the atmosphere) and a high salt content in the water.
Prevention:
- Frequent coating of paint, oil or grease on iron articles slows down the process of rusting.
- Galvanisation (depositing a layer of zinc on iron).
- Alloying (mixing iron with other metals).
Fact: The Iron Pillar at the Qutub complex in Delhi is more than \(1600\) years old. This pillar has not rusted even now, which is a testament to the advances in metal-making technology in the \(16th\) century in India.
2. Combustion:
Combustion is commonly known as burning. When a substance burns, it turns into ashes. The ashes have an entirely new chemical composition from that of the unburnt substances. It is an exothermic reaction.
The chemical process in which substances burn in the presence of air (oxygen) to release energy in the form of heat and light is called combustion.
Example:
Burning of a candle wick, paper, matchstick, fireworks, etc., are some of the common chemical changes that we see around us.


Burning of wood
Any substance that burns requires three essential elements: oxygen, heat, and fuel. Combustion is an exothermic process, which generally occurs with the release of heat to the surroundings as a product of the reaction.
1. Air: Oxygen is the essential element for any substance to burn. Oxygen is also called the supporter.
Activity: Oxygen is the supporter for combustion
For instance, light two candles inside a glass container. Cover one glass container with the lighted candle and leave the other uncovered.
Observation:
What happens to the flame of the burning candle in a closed container?
The burning wick of the candle inside the closed container turns off after a minute, while the other in the open container continues to burn. It turns off due to the absence of oxygen in a closed container, which stops the combustion process.

Burning a candle inside an open and a closed container
Result:
The main element of air that supports combustion is oxygen. Once there is no supply of oxygen, burning will not occur.
2. Fuel or Combustible substance: The substances that can catch fire in the presence of air are known as combustible substances. They can be solid, liquid or gases and must be flammable substances in nature, such as paper, wood, petrol, diesel, LPG, and CNG.
3. Heat: The lowest temperature at which a material catches fire is known as the ignition temperature. For example, let us compare wood and paper. Wood has a higher ignition level than paper. So when we burn both these at the same time, paper catches fire quickly than wood.
In the absence of any one element, burning will not occur. It requires three essential components: air, oxygen, and fuel, and this is known as the fire triangle. The final product of any combustible substance on complete burning produces ashes, carbon dioxide, water, water vapour, and smoke.
Let us now observe the reaction of magnesium ribbon when it burns. Magnesium ribbon produces a bright white light when burned and forms powdery ashes.
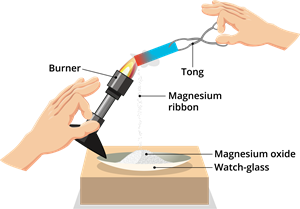
Burning of magnesium ribbon
We know that magnesium (\(Mg\)) is a metal. Metals, on reaction with oxygen, (\(O_2\)) form their oxides - similarly, magnesium forms magnesium oxide (\(MgO\)).
The ashes collected from the burning of the magnesium ribbon are magnesium oxide.
The burning of a substance is a chemical change, as it involves the formation of a new substance at the end of the reaction. Thus, the reaction in which there is a formation of an entirely new substance from that of the reactant(s) is known as a chemical reaction. These are permanent changes. Permanent changes are irreversible.
Mysteries of nature: In late evenings, you may have noticed insects in a field or garden that were generating light. These insects are known as fireflies, and a chemical reaction gives them their light. This kind of heat-free light generation in living things is known as bioluminescence.
Chemical reaction of baking soda with vinegar:
Sodium bicarbonate (sodium hydrogen carbonate) is commonly called baking soda. The chemical formula is \(NaHCO_3\). It is a base with a pH of \(8\). When reacted with acids, sodium bicarbonate produces effervescence of \(CO_2\) with other products, such as water and salt.
When baking soda, which is a base, is added to vinegar, an acid, they react together, and \(CO_2\) gas is evolved in the form of bubbles, producing a fizzing sound. The following reaction occurs,
\(NaHCO_3 + CH_3COOH \to CH_3COONa + H_2O + CO_2\)
\(\text{Baking soda} + \text{Vinegar} \to \text{Salt} + \text{Water} + \text{Carbon dioxide}\)
Test to confirm the released gas is carbon dioxide:
Pass the gas released into a test tube containing freshly prepared lime water. The solution in the test tube turns milky white.

Test for carbon dioxide
The escaping of the carbon dioxide from the reaction is confirmed by the change in the colour of lime water, leading to the formation of calcium carbonate, which appears milky.