Have you ever wondered how invisible substances impact the taste of your food, the fizzing in your beverages, and even the washing power of your washroom cleaner? Why does soap generally feel slippery?
Also, have you ever tasted a lemon and reacted by puckering your lips?
The secret agents hiding behind these mysteries are the acids and bases.

Types of tastes
In this chapter, you will discover the reason behind all the above questions, the different tastes in foods, drinks, colour changes in solutions and many more. These clever substances are always busy!
i. Acids are the sour super chemicals found in fruits like lemon, grapes, and oranges and even in your stomach.

Acid: Sour taste
ii. Bases are bitter and slippery cleansers found in leafy vegetables, spinach, soap, toothpaste, and baking soda.

Base: As a cleanser
Get ready to mix solutions, test, and explore the science behind the reactions, indicators, and pH value mysteries—where each solution has a story!
Nature - Our Science Laboratory:
You can not see acidity, you can not smell basicity, but with a drop of the right substances, colour shifts in that instance that were previously unseen become visible. These are acid-base indicators; one speaks not with words, but with colour! Let us learn more about different natural indicators.
The chemical substance that changes colour depending on the pH of the solution, indicating whether the solution is acidic, basic or neutral, is called an acid-base indicator.
1. Litmus as an indicator:
Can you believe that a piece of paper helps you to figure out whether it is an acid or a base when it just takes a slight dip in liquids? Yes, a special paper called Litmus.
Litmus paper is made from lichens, which are tiny plant-like organisms made of two separate organisms, a fungus and an alga. This partnership is helping with solution detection. And we use it mostly in the form of litmus paper, which is available in blue, red, and purple colours.

Lichens
Activity: Testing solutions with litmus paper
Materials Required:
-
Red and blue litmus paper
-
Lemon Juice
-
Vinegar
-
Soap solution
-
Baking soda solution
-
Lime water
-
Distilled water
-
Sugar solution
Instructions:
Step 1: Take the strips of red litmus paper.
Step 2: Take the sample solutions in separate test tubes and dip a red litmus paper into each test tube and observe the colour change.
Step 3: Repeat the same with the strips of blue litmus paper and observe the colour change.
Observation: The observations are tabulated as follows.
|
Samples
|
Red litmus
|
|
No change
|
|
|
No change
|
|
|
Blue
|
|
|
Blue
|
|
|
No change
|
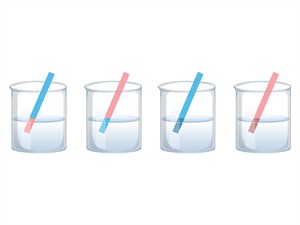
Litmus test: Acid, base and neutral solution
Result:
- If the liquid is sour and fizzy, it detects an acid, and the blue strip turns red.
- If the liquid is bitter and slippery, it detects a base, and the red strip turns blue.
- If the liquid is calm and chilled, it detects neutral, and both red and blue litmus remain unchanged.
| Litmus paper | Test with the acid substance | Test with the base substance |
| Blue litmus paper |
Blue \(→\) Red
|
Blue \(→\) Blue (No change)
|
| Red litmus paper |
Red \(→\) Red (No change)
|
Red \(→\) Blue
|
2. Red rose as an indicator:
Yes, you heard it right, Red Rose can help you figure out whether the solution is acid, base or neutral. It’s because of a natural chemical present in the petal which acts as an acid-base indicator.

Red rose
Red rose contains a natural pigment in their petals called anthocyanin. This pigment tends to change colour depending on the pH level of the liquids. Ready to make your indicator using a red rose!
Activity 1
Materials Required:
-
Red rose petals
-
Warm water
-
Small cups & spoon
-
Samples to test (lemon juice, soap solution, baking soda, distilled water)
Instructions:
Step 1: Take the red rose petals in a cup and, with the help of a spoon, gently mash the petals to release the colour.
Step 2: To the mashed petals, pour in some warm water and let it stay for 10 minutes, then strain the solution into a clean cup.
Step 3: Now, take a small amount of rose indicator in different cups and add samples to be tested in each cup, and observe the colour change.

Red rose extract
Observation:
The changes observed are listed below:
Lemon juice: Bright red
Pure water: Stays red
Baking soda: Greenish-blue or bluish
Soap solution: Yellow-green
Response of the red rose indicator depending on the nature of the solution is as listed below;
i. Acidic solution blends with red rose indicator and changes to a deep red.


Colour change of red rose: In acidic and basic solutions
ii. Basic solution blends with red rose indicator and changes to greenish, bluish, or even to yellowish, based on the strength of the basic solution.
iii. Neutral solution with red rose indicator stays in its natural red without changing.
3. China rose as an indicator:
The petals of china roses also act as a natural indicator. Anthocyanin, a water-soluble pigment found in china roses, changes colour when it reacts with an acid or a base.
Preparation of china rose solution:
Step 1: Take some petals of china rose in a cup
Step 2: And some warm water in that cup
Step 1: Take some petals of china rose in a cup
Step 2: And some warm water in that cup
China rose extract
Step 3: Let it rest aside until the water colour changes
Step 4: Filter the solution. The filtered solution will act as an acid-base indicator
China rose indicator changes the acidic solution to magenta (dark pink). It turns the basic solution to dark green colour and no colour change in neutral solutions.
Let us now test the solutions below for their acidity and basicity by adding the prepared solution to them. And observe the changes in their colour.
|
Samples
|
Colour change
|
|
Dark pink colour
|
|
|
Dark pink colour
|
|
|
Dark green colour
|
|
|
Remains unchanged
|
|
|
Dark green colour
|
|
|
Remains unchanged
|
Thus, in an acidic solution, the colour changes to deep pink or deep red. In the basic solution, the colour changes to green.
4. Turmeric as an indicator:
Can you believe that there is an ingredient straight out of the kitchen that acts as a detective to investigate the nature of the solutions? Yes, we have an agent yellow from our spices that's turmeric!
Ready to witness the role of turmeric as an acid-base indicator!
Activity: Let's create a unique card together to surprise your dad for Father’s Day
Materials Required:
-
Turmeric
-
Water
-
White paper and paintbrush
-
Soap solution
-
Lemon juice
Instructions:
Step 1: Mix the turmeric powder with water, and your golden yellow paint is ready.

Turmeric paste
Step 2: Apply turmeric paste on white paper and let it dry.

Paper painted with turmeric paste; turns red on painting with basic solution
Step 3: With the help of a paint brush or buds, write or paint your thoughts to make a card with lemon juice and soap solution on each paper, and watch what happens.
Observation:
What is the colour of the turmeric paste?
Ans: Yellow
Did any colour changes occur when painted with lemon juice?
Ans: No
Did any colour changes occur when painted with soap solution?
Ans: Turns reddish-brown
Can you name a solution which, when used, might change colour?
Ans: Baking soda solution
Conclusion:
Turmeric has a compound named curcumin, which is responsible for the colour change.
-
Acidic solution - Stays yellow
-
Basic solution - turns reddish-brown
- Neutral solution - Stays yellow
Interesting fact!