On a hot summer day, you and your friend are heading to a park, to a place, and to spend some time. After a joyful round of play, you all decide to find a spot where you can relax. You all noticed two benches unoccupied in the park.
One is a metal bench and the other is a wooden bench. You all decided to sit on a metal bench because it was attractive. While sitting on it, all felt that it was very hot.

A metal bench
Then, everyone decided to change the spot and sat on a wooden bench; it did not feel very hot. What could be the reason for the metal bench to be hot?
It is a special property of metals known as the conductivity of heat.

A wooden bench
Conductivity is the ability of a material to conduct heat or electricity.
Heat conductivity
i. Metals are good conductors of heat.
Activity: To find out if metals are good conductors of heat
- Take a wire made of aluminium or copper.
- Clamp this wire on a stand. Using wax, attach a pin to the free end of the wire.
- Heat the wire near the clamping point with a spirit lamp, candle or burner.
- What do you notice when some time has passed?
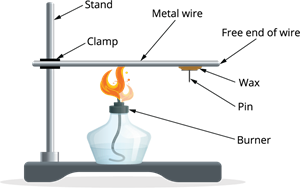
Heating a metal wire
Answer: The heat travels through the metal wire, melts the wax, and the pin falls down. This shows that metals are good conductors of heat.
Silver and copper are the best conductors of heat, while lead and mercury are relatively poor conductors of heat.
ii. Non-metals are poor conductors of heat; they do not transfer heat from one end to the other. Non-metals generally transfer heat poorly because they lack free electrons. Non-metals act as an insulator. But diamond conducts heat.
Activity: Heat transfer in materials
Instructions:
- Place a glass tumbler and fill it with hot water.
- Into the glass tumbler, immerse a metal spoon and a wooden spoon of the same length, and leave them for a few minutes.
- Later, carefully touch the upper end of each spoon.

Metal and wooden spoons immersed in water
Observation:
Even though both the metal and wooden spoons are placed in water of the same temperature and at the same time, the metal spoon feels hotter to the touch. This is because metal is a good conductor of heat, which allows it to transfer heat quickly. In contrast, wood has poor heat transfer properties, making it feel less hot.
As a result, most metals are considered good conductors of heat, while non-metals are poor conductors.
Applications of materials conducting heat:
- Copper, aluminium, iron, and stainless steel are used in the manufacturing of cooking utensils including pans, kettles, cookers, and pots.

Cooking utensil
- Aluminium, being a lightweight material, conducts heat quickly and evenly. It is cheaper than copper. Hence, it is widely used in the manufacturing of cooking utensils.
- Copper, steel, and aluminium are used in making heaters and electronic irons as they distribute heat quickly.
Electrical conductivity
Conductivity of electricity is the ability of a material to allow an electric current to pass through it.
Activity:
1. Make a circuit by using a battery, bulb and copper wires, as shown below.
2. Place a copper coin between the two free ends of the wire in the circuit, as shown below.
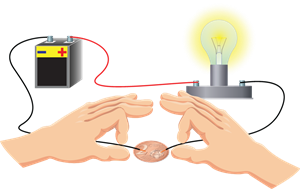
Electrical circuit
The bulb is glowing because metals are good conductors of electricity.
Metals contain free electrons, making them good conductors of electricity. These free electrons move easily through the metal, allowing it to conduct an electric current.
When the metal coin in the circuit is replaced by a non-metal, the bulb does not glow. Non-metals are poor conductors of electricity. Graphite (allotrope of carbon) conducts electricity because it has free electrons to allow current to pass through it.
Materials that do not allow electricity to pass through them are called poor conductors or insulators of electricity.
You have probably noticed that the wires that carry current in your homes have a coating of a rubber-like material called polyvinyl chloride (\(PVC\)).
Why are electric wires coated with PVC?
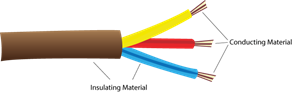
Electric wires coated with PVC
PVC is used to coat electric wires and cables to prevent electric shock since it is an insulator and a poor conductor of electricity.
Applications of materials conducting electricity:
- Copper is used in household electrical wiring and electrical circuits.
- Aluminium is used in wiring power lines.

Electronic kettle
- Copper, aluminium, and iron are used in the manufacturing of electrical appliances, including heaters, kettles, electric stoves, and fans.
- Gold and silver are used in circuit boards and microchips.
Sonority
The ability of a material to produce a loud sound or ringing sound when struck over is called sonority.
Activity: To determine the sonority of materials
Materials required:
- Hammer
- Steel plate
- Aluminium utensil
- Wooden block
- Rubber
Instructions:
Take a hammer and gently strike the steel plate, observing the sound produced. Repeat the same for materials such as aluminium utensil, wooden block, and rubber.
Observation:
A steel plate and an aluminium utensil produce a clear ringing sound, while a wooden block and a rubber produce a dull sound.
Conclusion:
i. Metals are sonorous because they vibrate uniformly when struck, producing a ringing sound. When stuck over hard surface, vibration travels through the metal and produces a clear sound.
ii. Non-metals are non-sonorous because they do not vibrate uniformly when struck on a hard surface, and they do not resonate. So, non-metals cannot produce a loud, ringing sound.

Metal bell
Application of sonority in daily life:
Metals are used widely because they are sonorous, durable and can be moulded to desired shapes - also, their ability to produce a resonant sound.
The practical applications of sonorous property in daily life and in industries are,
i. Musical instruments such as bells, drums, strings, and guitars.
ii. Bells, wind chains, electrical buzzers.

Wind chain
Reference:
https://1.bp.blogspot.com/-kpCdiNTyXf4/WMbnYWVLboI/AAAAAAAARQk/ih7_pQFGR-ckJDjRtkXVyVlbSx6Pv0jwgCLcB/s1600/CONDUCTINGINSULATINGWIRE.png
https://www.flickr.com/photos/daveparker/4984482207
https://pxhere.com/en/photo/1627149
https://www.flickr.com/photos/ell-r-brown/4153212203