Imagine the process of constructing a house. The initial step in this process involves sourcing essential raw materials, which serve as the foundation of the entire structure. Key materials like cement, bricks, and iron rods are crucial for ensuring the strength and stability of the house. Without these fundamental building blocks, the dream of a completed house would remain unattainable.

Construction of a building
In a broader context, this concept applies to everything in the universe around us. Just as a house is built from specific materials, all matter is composed of basic building blocks known as elements. These elements are the fundamental substances that combine in various ways to create the diverse array of materials and compounds that shape our world.
Let us learn in detail about the elements and their classification.
Element
The simplest form of matter is an element. Each element is made up of identical particles called atoms. Atoms are the simplest particles of an element that cannot be broken down in a chemical reaction.
Robert Boyle first used the term element. He was a supporter of the elemental essence of matter and the nature of the vacuum. Boyle's Law was his best-known work.

Robert Boyle
According to Antoine Laurent Lavoisier, an element is a fundamental type of matter that cannot be broken down into simpler substances by chemical reactions. A chemical element is a material that cannot be broken down into simpler forms using traditional chemical methods.
Example:
All the elements in the periodic table are examples of elements, such as oxygen, iron, gold, boron, and sulphur.
An atom is the smallest unit of a chemical element. Atoms are incredibly tiny, smaller than anything we can imagine or compare them to.
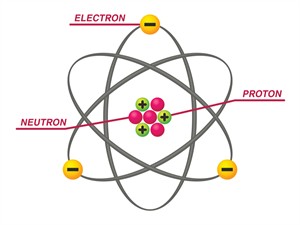
Structure of an atom
Subatomic particles (sub- means "smaller size") make up an atom. These particles are the,
- Proton (\(p^+\)), a positively (\(+\)) charged particle.
- Electron (\(e^–\)), a negatively (\(–\)) charged particle.
- Neutron (\(n^0\)), a neutral particle with no charge (\(0\)).
All the atoms in the periodic table are pure substances and can be termed as elements. The periodic table consists of \(118\) elements. Some of the elements and their symbols are listed below,
|
Element
|
Symbol
|
Element
|
Symbol
|
|
Oxygen
|
O |
Calcium
|
Ca |
|
Carbon
|
C |
Aluminium
|
Al |
|
Sodium
|
Na |
Sulphur
|
S |
|
Hydrogen
|
H |
Boron
|
B |
|
Iron
|
Fe |
Magnesium
|
Mg |
|
Chlorine
|
Cl |
Copper
|
Cu |
|
Nitrogen
|
N
|
Lithium
|
Li |
Each element has different physical and chemical properties. All the atoms in the periodic table are pure substances and have different physical and chemical properties.
Based on the physical and chemical properties, the classification of elements are,
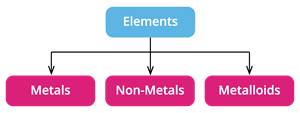


- Metals
- Nom-metals
- Metalloids

Classification of elements
1. Metals:
Metals are electropositive elements that donate electrons to form a stable configuration. A physical property can be observed and measured without altering the sample's chemical identity.
- Malleable (can be beaten into thin sheets)
- Ductile (can be drawn into wires)
- Lustre (shine) with silvery grey or golden yellow in colour
-
Sonorous (makes a ringing sound when hit)
-
Most of the metals are solid at room temperature, except mercury, which is liquid at room temperature.

Properties of metals
-
All metals are hard except lithium, sodium, and potassium (can be cut with a knife).
-
Metals have \(1\) to \(3\) electrons in their outermost shell.
-
Metals generally are good conductors of heat and electricity (they have free electrons in their outermost shell).
-
Metals have a high density (mass of unit volume of a material substance).
-
Metals have high melting and boiling points due to their strong metallic bonds, except for sodium and potassium, which have relatively low melting and boiling points.
The materials which generally possess the above properties are called metals.
2. Non-metals:
Non-Metals are electronegative elements that gain electrons to form a stable configuration.
- Non-metals are non-ductile and non-malleable in nature (as they are very brittle, they cannot be drawn into wires).
- Non-metals are soft except diamond (hard and shiny).

Properties of non-metals
- Non-metals generally are poor conductors of heat and electricity, except graphite, which conducts electricity.
- Non-metals can exist in all three states. But, mostly in gaseous state.
Example:
Oxygen, carbon, sulfur, hydrogen, phosphorus, nitrogen, halogens and noble gases, etc.
3. Metalloids:
They show the characteristics of both metals and non-metals and are known as metalloids. There are around \(8\) elements in the periodic table that are called metalloids.
Example:
Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te), Polonium (Po).
Physical properties:
- Metalloids are solid at room temperature.
- They can form alloys with other metals.
- Some metalloids (silicon and germanium) can behave as electrical conductors under specific conditions. Hence, they are called semiconductors.
- Silicon appears lustrous; however, it is neither malleable nor ductile (it is brittle, a characteristic of some non-metals).
- The physical properties of metalloids tend to be metallic, but their chemical properties tend to be non-metallic.
Reference:
https://www.freepik.com/free-vector/kids-overalls-helmets-building-house-happy-male-female-constructors-laying-bricks-using-construction-tools-flat-vector-illustration_16375297.htm