You might have looked at yourself in the mirror, glanced at your looks, and admired. Have you ever imagined what truly makes you? A closer look reveals a fascinating reality. Not just made of skin and bones. You are a collection of billions of tiny particles that are consistently interacting and moving.
Surprisingly, the same types of particles make up everything around us: the book in your hand, the pencil you use, the air you breathe, the food you eat, and even the stars in the galaxy. Everything is made of matter!

Rocks and a butterfly
If a rock and a butterfly are made of matter, why are they different? The incredible part of nature is that the arrangement of matter makes all the difference.
The study of the nature of matter explores how these tiny, invisible particles combine to form the things around us. It reveals the secret behind our world and ourselves.
In this chapter, we will learn about the nature of matter and how elements, compounds, and mixtures combine to form the various materials we encounter every day.
Mixtures:
Consider a bowl of fruit salad, which contains various types of fruits. These fruits can be distinguished by appearance and can also be separated by handpicking. This is an example of a mixture.

Fruit salad
In a mixture, the substances are combined in such a way that each retains its individual properties. The individual substances that are combined to make a mixture are known as components.
A mixture is a combination of two or more substances, composed of two or more elements, compounds, or both, that are physically combined.
Example:
Oil and water
Sand and water
Salads
Chalk and water
Sugar and water
The components of the mixture do not undergo any chemical reactions with each other. In some mixtures, the components are visible, while in others, they cannot be seen with the naked eye.
Based on the visibility and nature of components, mixtures are classified as follows:
- Uniform
- Non-uniform
1. Uniform mixture:
- All components of the mixture blend evenly.
- There are no visible separate barriers; the mixture appears to be uniform.
- It is a single-phase solution.
- Salt in water is the best example.
- Uniform mixtures are also called homogeneous mixture.
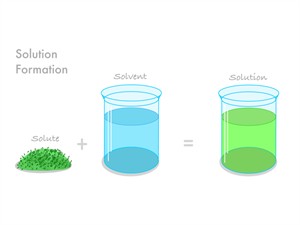
Uniform or homogeneous mixture
In homogeneous mixture, the components of the solution cannot be seen with the naked eye.
2. Non-uniform mixture:
- All components of the mixture are unevenly distributed.
- There are noticeable separate lines.
- There are two or more phases in the solution.
- Oil and water, sand and water, salad are examples.
- Non-uniform mixtures are also called heterogeneous mixtures.
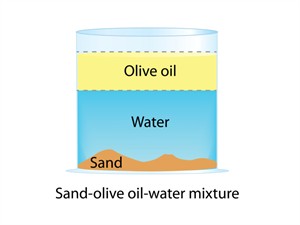
Non-uniform mixture
In a heterogeneous mixture, components are visible to the naked eye. It does not have a uniform composition, and the solute particles settle down.
Imagine a small room that appears empty. Is it truly empty? ! Air surrounds us, even though we cannot see it, and it occupies the empty room. This air is essential for survival.

Balloons filled with air
Have you ever wondered what air is made of? Is it a single substance or a combination of many components? Let us understand the composition of air.
Is air a mixture?
Air is a mixture. It is a combination of two or more substances that do not react chemically with each other.

Wind
Air is a uniform mixture of different gases, retaining their properties.
The main gases in the air are as follows:
- Nitrogen (~78%)
- Oxygen (~21%)
- Argon (~0.9%)
- Carbon Dioxide (~0.04%)
- Air also contains water vapour (moisture).
Activity: To test the presence of carbon dioxide in the air
Preparation of lime water:
- Take a test tube and fill it with water.
- Add a small quantity of calcium oxide (quick lime) to the water.
Quick lime reacts vigorously with water and leads to the formation of calcium hydroxide, releasing heat. The resulting solution is commonly known as lime water. Filter the solution, and it will appear as a colourless liquid.
Lime water turns milky:
- Place the lime water in a test tube in an open space.
- Stir the solution at regular intervals.

A clear solution of lime water turns milky
- You will observe that the lime water gradually turns milky white.
Reason: On placing the lime water in the open air, it reacts with carbon dioxide present in the air and turns milky white (due to the formation of calcium carbonate). Calcium carbonate appears as insoluble, tiny white particles. Hence, the solutions turn milky white, indicating the following reaction,
Calcium hydroxide + Carbon dioxide \(\to\) Calcium carbonate + Water
Types of mixture:
Mixtures can be of various types based on the nature and physical state of their components. The types of mixtures based on their physical states are as follows:
- Solids in a solid
- Solids in a liquid
- Liquids in a liquid
- Gas in a gas
- Gas in a liquid
1. Solids in a liquid: Salt solution and sugar solution
In solid in liquid mixture, the components are as follows:
Solute: Salt is the solute.
Solvent: Water is the solvent.
Solvent: Water is the solvent.

Salt solution
2. Gas in a liquid: Aerated drinks
In gas in liquid mixture, the components are as follows:
Solute: Carbon dioxide (gas) is the solute.
Solvent: Water (liquid) is the solvent.
Solvent: Water (liquid) is the solvent.

soft drinks
3. Solids in a solid: Alloys
Alloys are homogeneous mixtures of two or more metals, a metal and a non-metal, that can't be separated into their components using physical methods.

Objects made from Brass
However, since an alloy exhibits the properties of its constituent elements and may have a variable composition, it is still considered a mixture. Brass, for example, is made up of about \(30\%\) zinc and \(70\%\) copper.
4. Liquids in a liquid solution: Lemonade
In liquid in liquid mixture, the components are as follows:
Solute: Lemon extract is the solute.
Solvent: Water is the solvent.
Solvent: Water is the solvent.

Lemon extract in water
5. Gas in a gas solution: Natural gas and air
Air is an example of a gaseous mixture. Air is a gaseous mixture that is homogeneous in composition.
Reference:
https://commons.wikimedia.org/wiki/File:Blue_tiger_butterfly.jpg