Compounds are a form of matter created by combining two or more elements in a specific mass ratio. Chemical methods may be used to decompose it into its constituent components. It is a form of matter created by combining two or more elements in a specific mass ratio. To decompose it into its constituent components, we use chemical methods.
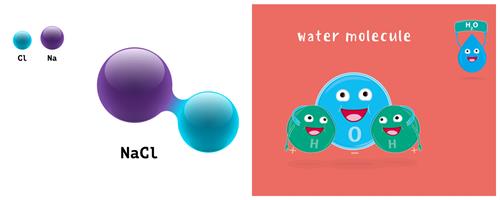
Salt and Water
A compound is a homogeneous mixture of two or more atoms chemically combined.
A chemical compound is formed when two or more distinct elements are chemically combined i.e. chemical bonds form between their atoms.
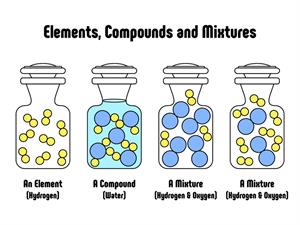
Pure substances and mixtures
Properties of Compounds:
- Two or more elements are chemically combined in a compound.
- The elements in a compound are present in a fixed mass ratio.
- Physical methods cannot isolate the constituents of a compound.
- The constituents of a compound lose their identities, i.e., a compound's properties vary from those of its constituent elements.
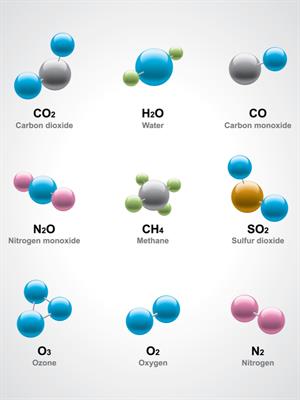
Compounds and molecules
Constituent elements in a compound:
- Water (\(H_2O\)) has three atoms: Two hydrogen (\(H\)) atoms and one oxygen (\(O\)) atom.
- Methane (\(CH_4\)) with five atoms: One carbon (\(C\)) atom and four hydrogen (H) atoms.
- Glucose (\(C_6H_{12}O_6\)) contains elements of carbon \(6\), hydrogen \(12\) and oxygen \(6\) combined to form a glucose molecule.
- Sodium chloride (\(NaCl\)) contains the two elements: sodium and chlorine atoms combined to form a sodium chloride molecule in a \(1:1\) ratio.
Activity: Comapring the nature of a mixture and a compound
Instructions:
Step 1: In a watch glass, take \(5.6 g\) of iron filings and \(3.2 g\) of sulfur powder. Mix them well in the watch glass and label this mixture as Sample \(A\).
Step 2: From Sample \(A\), take half of the mixture and transfer it to a china dish. Heat the mixture with constant stirring until it turns black.
Step 3: Once the mixture has cooled down, transfer it from the china dish to a mortar and grind it with the pestle. Finally, transfer the ground mixture to another watch glass and label it as Sample \(B\).
Observation:
i. Appearance:
Upon examining Sample \(A\), it is clear that the iron and sulfur components retain their distinct properties. The individual black and yellow particles of iron and sulfur are easily visible, indicating that this sample is a mixture of these elements.

Sample A: Iron and sulphur mixture
In contrast, Sample \(B\) presents a uniform black mass, which is iron sulfide. Sample \(B\) has a uniform texture and colour throughout, resulting from the heating of iron and sulfur together. Therefore, Sample B is a compound (iron sulphide).

Sample B: Iron sulphide
The reaction is as given below,
\(\text{Iron} + \text{Sulfur} \to \text{Iron sulphide}\)
ii. Magnetic effect:
On bringing a magnet close to Sample \(A\), the iron filings in the mixture are attracted to the magnet. Indicating that iron and sulfur can be separated through physical means.
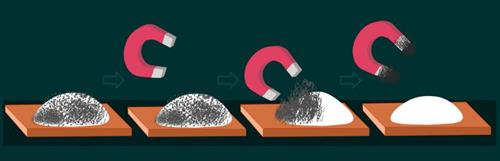
Magnetic separation on Sample A
In contrast, when a magnet is placed near Sample \(B\), it does not attract the components. This suggests that the compound has properties that are different from those of its component elements, iron and sulfur.
ii. Gas test:
To a small amount of Sample \(A\) in a test tube, add a few drops of dilute hydrochloric acid. A gas will evolve, and when you bring a burning splinter near the mouth of the test tube, the flame will produce a popping sound. The gas released is colourless and odourless, confirming the presence of hydrogen gas.
The reaction is as given below,
\(\text{Iron} + \text{dilute hydrochloric acid} \to \text{Iron chloride} + \text{Hydrogen gas}\)
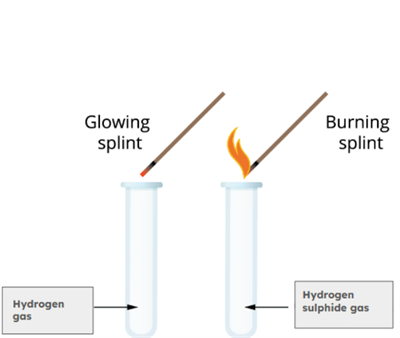
Gas test
Similarly, when you add a few drops of dilute hydrochloric acid to Sample \(B\) in another test tube, gas is also released, and it has a rotten egg smell. When a glowing splinter is placed near the mouth of this test tube, the flame continues to burn steadily.
\(\text{Iron sulphide} + \text{dilute hydrochloric acid} \to \text{Iron chloride} + \text{Hydrogen sulphide}\)
Thus, the above tests confirm that Sample A is a mixture of iron and sulfur, while Sample \(B\) is a compound of iron sulfide; both have different physical and chemical properties.