For instance, you are given two cups of tea. When you taste them, one seems to be sweeter than the other and appears to be dark in colour.
i. What conclusion do you draw from it?
The sugar content of the sweeter tea is higher than that of the other.
ii. What words can you use to express your observation?
You could say that the 'tea is stronger'.
A chemist, on the other hand, would say the tea is ‘concentrated’.
Concentrated and dilute solutions is a different type of classification of solutions. It expresses the relative concentration of two solutions in terms of the solutes present in a given amount of solvent.
Concentrated solution:
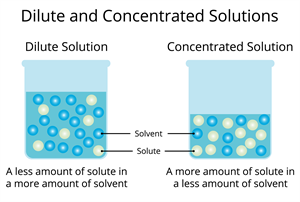
A concentrated solution contains large amount of solute dissolved in the given amount of solvent. The quantity of the dissolved solute is much higher than the quantity of the solvent.
Dilute solution:
A dilute solution contains small amount of solute dissolved in the given amount of solvent. The quantity of the dissolved solute is much lesser than the quantity of the solvent.
Types of solution based on solute
When two solutions with the same solute and solvent are compared, the one with a higher amount of solute per given amount of solvent is referred to as a concentrated solution. In contrast, the other is referred to as a dilute solution.
The distinction between dilute and concentrated solutions is a qualitative representation. It does not imply the amount of solute. This distinction is visible through physical characteristics such as colour, density, and so on.
Comparison of dilute and concentrated solutions:
The dark coloured solution of tea and copper sulphate is the concentrated, while the lighter coloured one is the diluted solution.

Concentrated solution

Dilute solution
In the above image, the solution marked as ‘(a)’ in both tea and copper sulphate is the concentrated solution, whereas ‘(b)’ is the dilute solution.
Effect of temperature on solubility:
Solubility refers to the maximum amount of a substance that can be dissolved in a given volume of solvent. Solubility in water is frequently expressed in \(gram/100mL\).
Activity: To determine the effect of temperature on solubility of solute
Instructions:
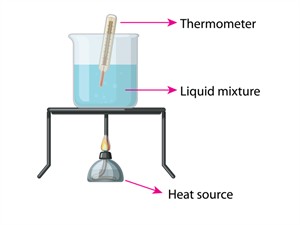
Step 1: Take \(50ml\) of water in a glass beaker, and using a laboratory thermometer measure its temperature.
Step 2: The initial temperature is \(20°C\), add a table spoon of baking soda and stir the solution well untill it gets completely dissolved.
Step 3: Continue adding baking soda, and stir continiously at one point baking soda remains undissolved. Now heat the solution in the glass beaker to \(50°C\) with constant stirring, and observe the changes.
Dissolution of baking soda in water
Step 4: Keep adding the baking soda untill the a few amount of baking soda remains undissolved. Then heat the solution to \(70°C\) with constant stirring, and bserve the changes.
Observation:
The undissolved baking soda in the solution gets dissolved when the temperature of the solution is increased from \(20°C\) to \(50°C\), and after adding extra baking soda some quantity of it remained undissolved. On increasing the temperature to \(70°C\) it got dissolved.
Interference from the experiment:
When the temperature of water is \(70°C\) it dissolved more baking soda than water at \(50°C\). The amount of baking soda dissolved at \(20°C\) is very low.
The solubility of the solute increases with an increase in the temperature. Also, when the temperature is increased, the saturated solution can also behave as an unsaturated solution.
The solubility of solute is governed by three major factors:
- Nature of the solute and solvent
- Temperature
- Pressure
1. Nature of the solute and solvent:
Solubility is greatly influenced by the nature of the solute and solvent. Although water dissolves a wide range of ionic and covalent substances, it does not dissolve everything. The solution in which water acts as a solvent is known as aqueous solution.
When predicting solubility, scientists frequently use the phrase "like dissolves like". This expression means that dissolving occurs when the solvent and the solute have similarities.
For instance, common salt is a polar compound that dissolves easily in a polar solvent such as water, the common salt will not get dissolved in non-polar solvents such as oil.
In non-polar solvents, non-polar compounds are soluble. For example, fat dissolved in ether.

Iodine in a) carbon tetrachloride b) water
Iodine is a non-polar substance, it dissolves in carbon tetrachloride. While it does not dissolve in the polar solvent water.
Non-polar compounds, do not dissolve in polar solvents, and polar compounds do not dissolve in non-polar solvents.
2. Temperature:
Solubility of solids in liquid:
In general, the solubility of a solid solute in a liquid solvent increases as the temperature rises.
Dissolution is the process in which solid solute dissolves completely in a solvent to form a solution.
The solubility of the solute increases with an increase in the temperature. Also, when the temperature is increased, the saturated solution can also behave as an unsaturated solution.
The kinetic energy of the particles increases on increasing the temperature.
- When the temperature increases, particles gain more energy and move quick and dissolves faster.
- When the temperature decreases, particles lose energy and move more slowly. and dissolves slower.
Example:
Sugar dissolves more readily in warm water than in cold water.
Based on the change in temperature of the process,
- Solubility increases with absorbing heat, in an endothermic process.
- Solubility decreases with releasing heat, in an exothermic process.
Let us learn about the solubility of gases in liquid and the effect of pressure on solubility in the upcoming theory.
Reference:
https://stocksnap.io/photo/tea-teabags-NYSS2K287L
https://www.pxfuel.com/en/free-photo-eqkhd