Have you ever wondered why salt disappears when added to water and stirred well? The crystals vanish, yet the salty taste remains. The reason is that salt dissolves in water, forming a solution.
The world is full of solutions: the coffee you drink, the blood in your veins, the air around us, the lemonade, and even medicines. The world of solutions tells a story of molecular friendship and cooperation between particles.

Solutions
Let us learn how substances interact, combine and dissolve to form a solution and understand its nature.
Types of mixture:
The components of the mixture do not undergo any chemical reactions with each other. In some mixtures, the components are visible, while in others, they cannot be seen with the naked eye.
Based on the visibility and nature of components, mixtures are classified as follows:
- Uniform
- Non-uniform
1. Uniform mixture:
- All components of the mixture blends evenly.
- There are no visible separate barriers in the mixture appears to be uniform.
- It is a single-phase solution.
- Salt in water is the best example.
- Uniform mixtures are also called homogeneous mixture.

Uniform mixture
In homogeneous mixture, the components of the solution cannot be seen with the naked eye.
2. Non-uniform mixture:
- All components of the mixture are unevenly distributed.
- There are noticeable separate lines.
- There are two or more phases in the solution.
- Oil and water, sand and water, salad are examples.
- Non-uniform mixtures are also called heterogeneous mixtures.
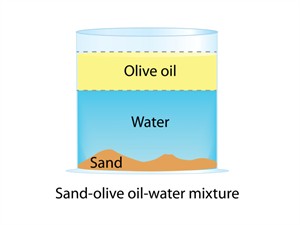
Non-uniform mixture
In a heterogeneous mixture, components are visible to the naked eye. It does not have a uniform composition, and the solute particles settle down.
Solute:
A solute is the substance that dissolves in the solvent to form a solution, which is a homogeneous mixture. It is generally present in a smaller quantity, but the key feature of a solute is that it gets dissolved, not its amount.

Solute
Solute can exist in any state of matter:
- Solid: Salt, sugar
- Liquid: Acetic acid
- Gas: Carbon dioxide, oxygen
Solvent:
A solvent is a substance that dissolves the solute particles to form a solution. Mostly, the solvent is present in larger quantities compared to the solute.
Water is a universal solvent and it is a polar solvent. The solutions which contain water as solvent is called as aqueous solutions.

Universal solvent: Water
Some substances do not dissolve in water. As a result, non-polar solvents such as ethers and benzene are used to dissolve such substances.
Alcohols, although polar, can also dissolve certain substances because they have both polar and non-polar characteristics.
Solution:
Whenever we drink lemonade juice, it tastes the same throughout. It shows that particles of sugar or salt are evenly distributed in the solution (juice).
A solution is a homogeneous mixture that has the same or similar properties throughout the mixture. Consider the example of saltwater.
A solution is a homogeneous mixture of two or more substances, appears to be uniform.
- A solute is a part of a solution that is present in a smaller quantity by weight.
- A solvent is a variable that is present in a greater quantity.
On adding solute into the solvent, we get a solution,
\(Solution = Solvent + Solute\)
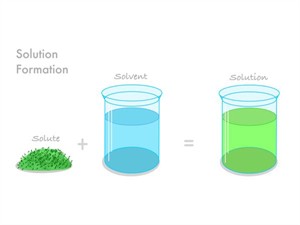
Formation of solution
A solution must have at least two components (a solute and a solvent). Binary solutions are those that consist of one solute and one solvent (two components).
Activity: To determine the amount of solute that can be dissolved in water
Instructions:
- Take a clean glass tumbler and fill it with water until it is half full.
- Add one spoonful of sugar to the water and stir well until it dissolves completely.
- Gradually continue adding spoonfuls of sugar while stirring constantly.
Observe how many spoonfuls of sugar you add before it stops dissolving.
Observation:
When sugar is first added to water, it dissolves completely, creating a solution. However, after adding more spoons beyond a certain point, sugar can no longer dissolve fully and instead settle at the bottom of the container.
Result:
The sugar that remained undissolved indicates that water can no longer dissolve sugar, because it has reached its limit. The solute particles stops dissolving and settles down at the bottom called saturated solution.
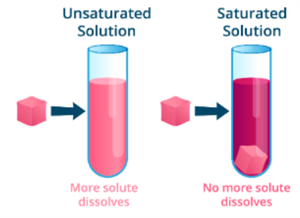
Saturated solution:
A saturated solution is one in which no more solute can be dissolved in a specific amount of solvent at a given temperature.
Example:
At \(25° C\), \(36 g\) of sodium chloride in \(100 g\) of water forms a saturated solution. If some more sodium chloride is added after this, it will be undissolved.
Saturated and unsaturated solution
Unsaturated solution:
At a given temperature, an unsaturated solution contains less solute than a solvent can dissolve in a given temperature.
Example:
At \(25° C\), \(10 g\), \(20 g\), or \(30 g\) of sodium chloride in \(100 g\) of water forms an unsaturated solution.