Have you ever observed the moment when you twist open a bottle of soda? A fizzing sound, countless bubbles rush out into the air. These lively bubbles are actually tiny carbon dioxide gas dissolved in the sweetened liquid. As the pressure in the sealed bottle is released, the gas rapidly comes out.


Gas bubbles on the surface
The solubility of gases in liquids explains the formation of fizzy bubbles and supports the life of aquatic organisms underwater.
Solubility of gases:
Many gases are dissolved in water. Oxygen dissolves in water only in small quantity, though it is present in less quatity this dissolved oxygen helps in the survival of aquatic life such as plants, fishes, and other organisms.
The solubility of gases is the ability of a gas to dissolve in a liquid to form a solution under certain conditions of temperature and pressure.


Aquatic species in water
The mixture of gases in water results in the formation of homogeneous mixture (uniform mixture). The gases such as carbon dioxide and oxygen dissolves completely in the liquid and distributed evenly in water to form a solution.
In contrast, if the bubbles are visible and not dissolved completely it is an heterogeneous mixture (non-uniform mixture). As long as the gas remains completely dissolved it is uniform mixture.
Among common gases, ammonia is the most soluble.
Factors affecting solubility of gases in liquid:
i. Temperature:
Do you know why water bubbles form when water is boiled?
With increasing temperature, the solubility of gases in liquids decreases causing it to evaporate forming bubbles.
In general, water contains dissolved oxygen. When water is heated, the solubility of oxygen in water decreases, allowing oxygen to escape in the form of bubbles.
Aquatic animals thrive in colder climates because the water contains a higher concentration of dissolved oxygen. This demonstrates that oxygen is more soluble in water at low temperatures.
ii. Pressure:
Only when gas is soluble in a liquid, the effects of pressure is observed. The solubility of a gas in liquid increases as pressure is increased.
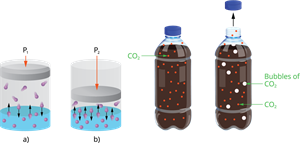

Effect of pressure on solubility
Example:
Carbonated beverages, i.e. soft drinks, household cleaners containing an aqueous solution of ammonia, formalin -aqueous solution of formaldehyde, and so on, are common examples of gas solubility in liquids.
Density of matrials
In a beaker filled with water, an iron ball and a wooden cork is dropped simultaneously. Now, what will you observe? From the picture, it is observed that the cork floats and the iron ball sinks.


Floating and sinking of objects
When a single drop of water is dropped into the oil, it sinks. However, if one drop of oil is dropped into water, it floats and builds a layer on the surface. Through this, we can say, some oil are denser than water.


Oil floating on water
Water is denser than cooking oil and castor oil, even though these oils appear to be denser than water. Castor oil has a density of \(961 kg/m^3\), whereas water has a density of \(1000 kg/m^3\) or \(1g/cm^3\).
Density is defined as the amount of mass present in a unit volume of a substance.
Materials with a higher density are referred to as denser, while those with a lower density are called less dense or lighter.
The density of a substance may be expressed mathematically using the formula:
The SI unit of density is \(kg/ m^3\) and the CGS (Centimetre Gram Second) unit is \(g/ cm^3\). The common unit of density for liquids is expressed as \(g/mL\).
The density of a substance is independent of its shape or size.
Reference:
https://commons.wikimedia.org/wiki/File:CNX_Chem_13_01_SuperSat.jpg
https://media.easy-peasy.ai/27feb2bb-aeb4-4a83-9fb6-8f3f2a15885e/77fdae37-0aec-40fa-9a0d-dbed63d52f1b.png