John Dalton, born in 1766 in England, was a pioneer in understanding matter. In 1808, he proposed the atomic theory, which explained that all matter is made of tiny, indivisible particles called atoms. Dalton’s work laid the foundation for modern chemistry.

John Dalton
Postulates of Dalton's atomic theory:
-
All matter is made of tiny particles called atoms.
-
Atoms cannot be created or destroyed in chemical reactions.
-
Atoms of the same element are identical in mass and properties.
-
Atoms of different elements have different masses and properties.
-
Atoms combine in simple whole-number ratios to form compounds.
- The relative number and kinds of atoms are constant in a given compound.
Atoms:
The smallest unit of an element. Composed of subatomic particles:
- Protons (\(p^+\)) – Positive charge
- Neutrons (\(n^0\)) – No charge
- Electrons (\(e^-\)) – Negative charge
Nucleus contains protons and neutrons; electrons orbit in shells.
We know that atoms are too small, but what is the size of an atom? Now, let us compare the size of atoms with some materials.
| S. No | Radii (in meter) | Example |
| 1 | \(10^{-10}\) | Hydrogen atom |
| 2 | \(10^{-9}\) | Water molecule |
| 3 | \(10^{-8}\) | Molecule of haemoglobin |
| 4 | \(10^{-4}\) | Grain of sand |
| 5 | \(10^{-2}\) | Size of an ant |
| 6 | \(10^{-1}\) | Watermelon |
Elements:
Substances that cannot be broken down into simpler substances by chemical methods.
Symbols of elements:
- Jöns Jacob Berzelius, a Swedish chemist, proposed that element symbols be made up of one or two letters from the element's name.
- But John Dalton was the first scientist to use the symbols for elements in a very specific sense. Below we see the symbols of the atoms proposed by John Dalton.
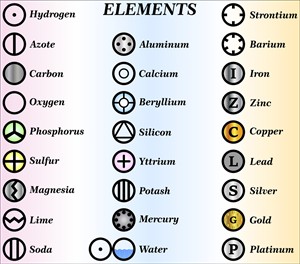
Dalton's symbols for elements
We can see that each element has a name and a unique chemical symbol. Some elements symbols are made up of the first letter of the name and a letter that appears later in the name.
Example:
i. Hydrogen - \(H\)
ii. Magnesium - \(Mg\)
But some elements named after another language like Greek and Latin, so those elements would be different from others.
Example:
i. The symbol of iron is \(Fe\) from its Latin name Ferrum (\(Fe\)).
ii. Potassium symbol is \(K\) from Kalium (\(K\)).
iii. Sodium is \(Na\) from Natrium (\(Na\)).
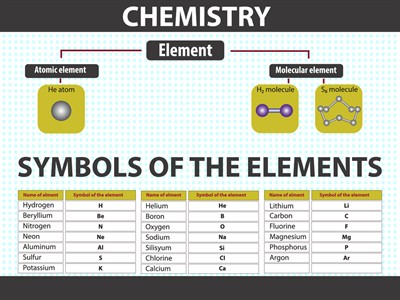
Symbols of the elements
In modern times, the International Union of Pure and Applied Chemistry (IUPAC) is responsible for officially approving the names of chemical elements.
We learned that matter is made up of atoms and molecules, and that each element is represented by a unique symbol. Next, we’ll explore atomic mass, molecules, and ions to understand how atoms combine to form different substances.