Have you ever wondered what everything around us is made of, from the air we breathe to the phone in your hand? Whether it’s the food we eat or the clothes we wear, everything is made up of matter.
For example, when water boils and turns into steam, nothing is lost! The same matter simply changes its form. This simple idea leads to two big concepts in chemistry:
-
Everything in the universe is made of matter.
-
Atoms are the smallest particles of elements that cannot be broken down chemically.
These ideas form the basis of all chemical studies and discoveries.
Historical background:
These fundamental ideas about matter and atoms were not discovered overnight. Long before modern science, ancient Indian and Greek philosophers had already begun questioning what matter is made of and how small its particles could be.
-
Around 500 BC, the Indian philosopher Maharishi Kanad proposed that if we go on dividing matter, we will ultimately get very small particles called Parmanu (atoms).
-
Around the same time, Greek philosophers Democritus and Leucippus suggested that matter is made of tiny indivisible particles called atoms (meaning “indivisible”).
However, these were only philosophical ideas, not proven by experiments. It was only after the 18th century that scientists began experimenting and proved the existence of atoms. Later discoveries showed that atoms can be further divided into electrons, protons, and neutrons.
Laws of chemical combination:
Chemistry deals with matter and the changes it undergoes, which follow specific rules called the laws of chemical combination, proposed by Antoine Lavoisier and Joseph Proust.
The laws of chemical combinations are
- Law of conservation of mass
- Law of constant proportions or law of definite proportions
Law of conservation of mass:
“The mass in an isolated system can neither be created nor destroyed but can be transformed from one form to another”.
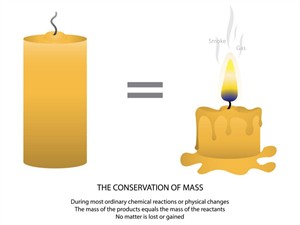
Law of conservation of mass
Law of constant (definite) proportion:
The elements are always present in definite proportions by mass in a chemical substance.
In water (\(H_2O\)), the ratio of the mass of hydrogen to oxygen is always \(1:8\), whether it is tap water, rainwater, or distilled water.
So, if \(9\ g\) of water is decomposed, it will always give \(1\ g\) of hydrogen and \(8\ g\) of oxygen.
The laws of chemical combination explain how matter interacts and transforms in predictable ways, laying the foundation for understanding chemical reactions. Building on this, we will explore Dalton’s atomic theory and the language of chemistry in the next session.