Chemical formulae:
A chemical formula is a symbolic representation of a compound showing the elements present and the number of atoms of each element. Before writing a chemical formula, it is important to understand the symbols and valency of elements. Just as different organisms have different capacities (for example, humans have two arms while an octopus has eight), atoms also have different combining capacities; this property is called valency.
Valency:
Valency is the combining capacity of an element. It tells us how many atoms of one element can combine with atoms of another to form a compound.
For example, hydrogen has a valency of \(1\), oxygen has \(2\), and nitrogen has \(3\).
Valency helps in writing correct chemical formulae of compounds.
Rules for writing a chemical formula:
-
Balance valencies or charges of combining elements or ions.
-
Write the symbol of the metal (positive ion) first, followed by the non-metal (negative ion).
-
Use brackets when more than one group of ions is needed.
Let us now see a few examples to understand how to write a chemical formulae for a compound.
Formulae of simple compounds:
The constituent elements and their valencies are written down in the chemical formulae for compounds, as shown below.
The valencies of the combining atoms must then be crossed.
We know the symbol for hydrogen \(H\) and oxygen \(O\).
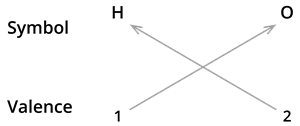
Formula: \(H_2O\)
2. Formula of carbon tetrachloride.
We know the symbol for carbon \(C\) and chlorine \(Cl\).
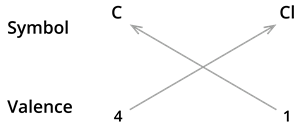
Formula: \(CCl_4\)
Note: The ions' charges are also not specified in the chemical formula.
3. Formula of magnesium chloride:
We know the symbol for magnesium \(Mg\) and chlorine \(Cl\).
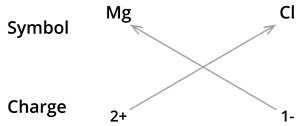
Formula: \(MgCl_2\)
4. Formula of aluminium oxide:
We know the symbol for aluminium \(Al\) and oxygen \(O\).
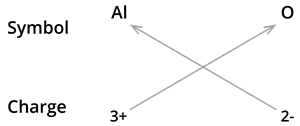
Formula: \(Al_2O_3\)
5. Formula of calcium oxide:
We know the symbol for Calcium \(Ca\) and oxygen \(O\).
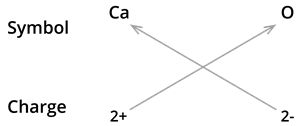
Formula: \(CaO\)
Note: The valencies of the two elements are the same in this case. You could come up with the formula \(Ca_2O_2\). However, we have shortened the formula to \(CaO\).
6. Formula of calcium hydroxide:
We know the symbol for calcium \(Ca\) and hydroxyl groups \(OH\).
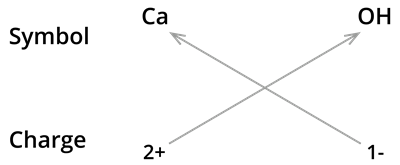
Formula:
Note:
-
The formula of calcium hydroxide is and not \(CaOH_2\). We use brackets when we have two or more of the same ions in the formula. Here, it indicates that there are two hydroxyl \((OH)\) groups joined to one calcium atom \(Ca\). And also, remember that brackets are not needed if only one ion is present.
-
It is worth noting that the charges on the ions are not mentioned in the formula.
Molecular mass:
The molecular mass of a substance is the sum of the atomic masses of all the atoms present in one molecule. It is expressed in atomic mass units (u).
Example: Molecular mass of water,
\(H_2O\) \(= (2 × 1) + (1 × 16)\)
\(= 18 u\)
Formula unit mass:
- For ionic compounds, we use the term formula unit mass instead of molecular mass.
- It is the sum of the atomic masses of all atoms in the formula of an ionic compound.
Example: Formula unit mass of sodium chloride,
\(NaCl\) \(= (1 × 23) + (1 × 35.5)\)
\(= 58.5 u\)
By understanding these concepts, we can explain how atoms form molecules and compounds, represent them with chemical formulae, and calculate their molecular or formula unit masses, building a strong foundation for exploring chemical reactions and equations.