Everything in this universe is made up of material, which scientists have termed "Matter".
Matter's Physical Nature:
Matter contains "\(n\)" number of particles. The particles of matter are very small, which is beyond our imagination.
We can distinguish the characteristics of particles of matter into three types.
1. Particles of matter have space between them.
Take a beaker with water and dissolve some salt with the help of a glass rod. Wait for a few seconds.
We already know that these particles are very tiny in nature. When we dissolve salt in water, the particles of salt get into the spaces between the particles of water. Thus, the water level is not raised.

Dissolving salt in water
2. Particles in the matter are continuously moving.
To see this, drop a little blue ink into a bowl of water. Without stirring, the blue colour slowly spreads on its own. This shows that the particles of water and ink keep moving and mix by themselves.

Diffusion of ink
3. Particles of matter attract each other.
The particles of matter attract each other due to the various forces acting between them. These forces keep the particles together. The strength of the forces of attraction varies from one kind of matter to another.
States of matter
We can classify the matters into three types: these are
- Solid state
- Liquid state
- Gaseous state
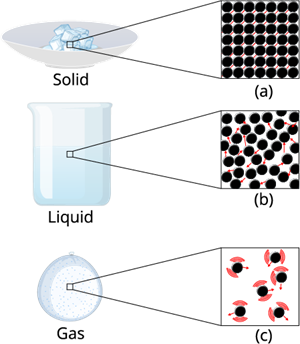
1. Solid state:
The objects that have a definite shape, distinct boundaries, and a fixed volume are called solid objects, or they are in a solid state.
The intermolecular force in the solid is the strongest force compared to the liquid and gas. This strongest intermolecular force makes the solid matter denser and gives it a certain shape and structure.
2. Liquid state:
A liquid is an intermediate phase between a solid and a gas. It has a fixed volume but no fixed shape. A liquid can take the shape of any container it is kept in without changing its volume.
The intermolecular force in a liquid is not as high as that of a solid, but it's higher than that of a gas.
Intermolecular forces of matter
3. Gaseous state:
A gas is a state of matter in which particles are very far apart and move freely in all directions. It has neither a fixed shape nor a fixed volume. A gas expands to fill the entire space of any container it is kept in.
The intermolecular force in the gaseous substance is weaker compared to that in the liquid and solid. This weaker intermolecular force allows the gaseous matter to move freely in the air.
Transformation of states
1. Melting / Fusion: When the temperature increases, solid particles gain energy and break their fixed positions to form a liquid.
2. Vaporisation: The process by which a liquid converts its physical state to a gaseous state is known as vaporisation.
3. Condensation: The process of a gas converting its physical state to a liquid state is known as condensation. This is also called reverse vaporisation.
4. Freezing/Solidification: On cooling, liquid particles lose energy, move slowly, and get tightly packed to form a solid.
5. Sublimation: Some solids directly change into a gas on heating because their particles get enough energy to overcome attraction.
6. Deposition: When gas cools rapidly, its particles lose energy and directly form a solid without becoming liquid.
Transformations of states
Boiling point:
The temperature at which a liquid begins to boil at atmospheric pressure is known as its boiling point.
Melting Point:
The temperature at which a solid substance melts to become a liquid at atmospheric pressure is called the melting point of that substance.
Celsius and Kelvin:
The SI unit of temperature is Kelvin (K). Celsius (°C) is not an SI unit, but it is commonly used in daily life.
We use the symbol \(°C\) to represent the Celsius; similarly, we use \(K\) for Kelvin.
When the Celsius is \(0°\), the Kelvin will be \(273.15K\).
That is, .
Celsius to Kelvin:
To convert the temperature on the Celsius scale to the Kelvin scale, we have to add 273.15 to the given temperature.
Kelvin to Celsius:
To convert the temperature from the Kelvin scale to the Celsius scale, subtract \(273.15\) from the given temperature.
Absolute Zero:
The temperature at which there is no motion and no heat remains in the substance, and all the particles will freeze their motion.
Absolute zero is the temperature at which a thermodynamic system has the lowest energy. It corresponds to \(-273.15\) °C (Celsius)or \(-459.67\ \)°F (Fahrenheit).
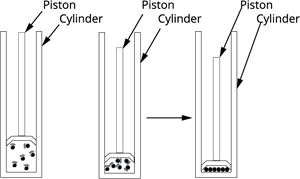
Effects of pressure on states:
Solids have particles that are tightly packed, so applying pressure does not change their shape or volume.
Liquids have particles that are close but can move slightly, so pressure changes their volume very little.
Gases have particles that are far apart and move freely, so they can be easily compressed or expanded by changing the pressure.
Therefore, solids are almost incompressible, liquids are slightly compressible, and gases are highly compressible.
Effects of pressure
Evaporation:
Evaporation is the process by which molecules at the surface of a liquid gain enough energy to change into the gaseous state, below the boiling point.
Evaporation is a phenomenon acting on the surface. The evaporation process increases with the following:
1. An increase in surface area:
If the surface area of the liquid layer is increased, the rate of evaporation increases.
2. An increase in temperature:
When the temperature of the liquid increases, the particles in the liquid get enough kinetic energy to reach the liquid to vapour state.
3. A decrease in humidity (the amount of water vapour present in air):
When the amount of water in the air is significantly high, it decreases evaporation.
4. An increase in wind speed:
We might have seen that when the wind speed is high, the wet clothes will dry faster. As the wind speed increases, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surroundings.

Evaporation