Everything in this universe is made up of material which scientists have termed as "Matter".
Take a moment to look at our surroundings. We see a variety of things with different shapes, sizes and textures. The water we drink, the food we eat, the clothes we wear, and the air we breathe every day, each of these things have their own mass and volume.
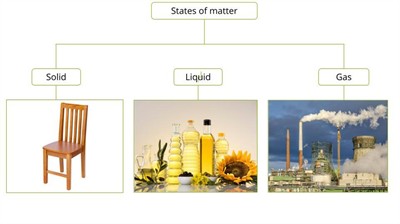
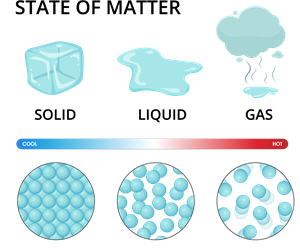
Different states of matter
According to our ancient Indian philosophers every living or non-living, thing was made up of five basic elements, named, the Panch Tatva. The five basic elements are Air, Earth, Fire, Sky and Water.
But our modern-day scientists have proposed two types of classification of matter based on their physical properties and chemical nature.
Let us learn about matter based on its physical properties and chemical nature in details.
Matter's Physical Nature:
Matter contains \(n\) number of particles. The particles of matter are very tiny which is beyond our imagination. The particles of matter has mass and occupies space.
Matter consists of atoms. Atom is the smallest particle that we cannot see through nacked eyes, even on the standard microscopes. Science introduced a technology to identify the structure of atoms: SEM (Scanning Electron Microscope) and TEM (Tunneling Electron microscope) that uses electricity to map atoms.
An atom consists of particles of neutron, electron, and protons in which neutrons and protons are present in the nucleus of the atom and electrons are present outside of the nucleus.
An atom consists of particles of neutron, electron, and protons in which neutrons and protons are present in the nucleus of the atom and electrons are present outside of the nucleus.
Let see an example to understand this clearly.
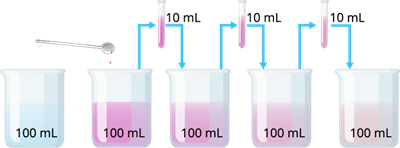
Potassium permanganate dissolution in water
The above experiment shows that just a few crystals of potassium permanganate can colour a huge volume of water. So we desired that there must be millions of very tiny particles in just one crystal of potassium permanganate, which keep on dividing themselves into smaller and smaller particles.
The particles of matter are very small they are very small beyond our thinking.
- Everything in this universe is made up of matter.
- Generally, matters occurs in solid, liquid and gas.
- Matter consists of tiny particles.
The characteristics of particles of matter are,
1. Particles of matter have space between them.
2. Particles in a matter are continuously moving.
3. Particles of matter attract each other.
1. Particles of matter have space between them.
Take a beaker with water and dissolve some salt with the help of a glass rod. Wait for few seconds.
We already know that these particles are very tiny in nature. When we dissolve in water, the particles of salt get into the spaces between particles of water. Thus, the water level is not raised.
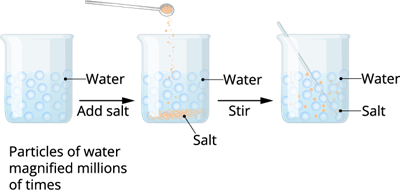
Dissolving salt in water
And, think about what will happen if you add the medium size stones instead of particles.
If you think that the water level will raise, then, you're right.
When we dissolve salt in water, the particles of salt get into the spaces between particles of water. This experiment clearly shows that there is enough space between the particles of matter.
Spacing in particles
Similarly, when we make lemonade (lemon juice), tea or coffee, we can observe the same principle.
2. Particles in the matter are continuously moving.
Particles of matter moves continuously, but slowly. To clarify this concept, let us do one experiment.
Step 1: Take a glass or bowl filled with water.
Step 2: Drop of red ink slowly along the sides of the bowl.
Leave it undisturbed for a few minutes. And, note down what you see after a couple of minutes.
You could see that the ink is spreading to water slowly. This proves that the particles in a matter are moving.

Moving particles
In another case, if we increase the temperature, the motion of the particles will also increase; therefore, their kinetic energy also increases.
Therefore, we can say that with increase in temperature, the kinetic energy of the particles also increases.
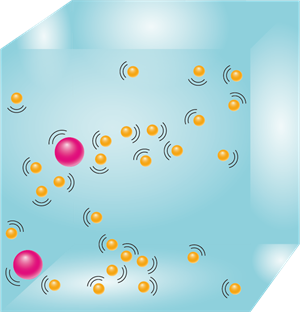
3. Particles of matter attract each other.
The particles of matter attract each other due to the various forces acting between them. These forces keep the particles together. The strength of the forces of attraction varies from one kind of matter to another.

Attracting particles
States of matter
A building or house is constructed with the help of bricks, stones and other solid materials.
And, the water we drink, the lake, and the sea, etc. are in the liquid form. Then, the air we breathe and the gases in our atmosphere are in a gaseous stage.
Therefore, generally, we can classify the matters into three types these are
And, the water we drink, the lake, and the sea, etc. are in the liquid form. Then, the air we breathe and the gases in our atmosphere are in a gaseous stage.
Therefore, generally, we can classify the matters into three types these are
- Solid state
- Liquid state
- Gaseous state
States of matter
In upcoming theory, we shall learn about these three states of a matter in greater details.