Objective:
In this exercise, we will learn the method to convert the Celsius to Kelvin and vice versa.
Celsius and Kelvin:
The Kelvin and Celsius are the SI units used to measure the temperature of objects.
We use the symbol \(C\) to represent the Celsius; similarly, we use \(K\) for Kelvin.
When the Celsius is \(0°\), the Kelvin will be \(273.15K\).
That is,
Relation between the Celsius and Kelvin:
The below formula explains the relationship between the Celsius and Kelvin.
Here T denotes the temperature, K denotes the Kelvin and C denotes Celsius of a matter.
Now, let us learn how convert the temperature Celsius to Kelvin and vice versa.
Celsius to Kelvin:
To convert the temperature on the Celsius scale to the Kelvin scale, we have to add 273.15 from the given temperature.
Kelvin to Celsius:
To convert the temperature from Kelvin scale to the Celsius scale, subtract \(273.15\) from the given temperature.
Absolute Zero:
The temperature at which there is no motion and no heat remains in the substance, and all the particles will freeze their motion.
Absolute zero, temperature at which a thermodynamic system has the lowest energy. It corresponds to \(-273.15\) °C (Celsius)or \(-459.67\ \)°F (Fahrenheit).
Important!
The minimum value of Kelvin is \(0\). Kelvin scale cannot be negative, because, at the zero Kelvin the particles in the substances will freeze their motion. Therefore, Kelvin scale is impossible to be negative one.
Effects of pressure on states:
We have studied that the matter can change its state due to the effects of the temperature. But, what will happen if we change the pressure? If we start putting pressure and compress a gas enclosed in a cylinder,what will be consequences?
How will the matter will react due to change in pressure?
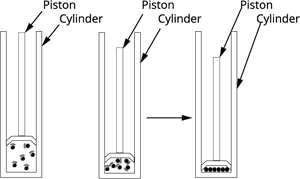
Effects on pressure
The effects of pressure are negligible on solids substances and more on liquid and most on the gases.
When the pressure is applied to a container of gas, the gas particles come together. When the pressure is increased that reduce the kinetic energy of the particle.
When pressure is increased further the gas-particle get near to each other, and then converted into the liquid form.
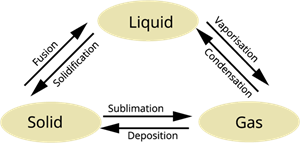
Interconversion of the three states of matter
Therefore, whenever we apply pressure to the gas particles, it converts its state to liquid.
Evaporation:
In the evaporation process, when the heat is applied, the water changes into water vapour.

Water cycle
Evaporation is the process by which molecules at the surface of a liquid gain enough energy to change into the gaseous state, below the boiling point
Now we get an idea about the evaporation. Let's move on to what are the things that can affect the evaporation.
Evaporation is a phenomenon acting on the surface. The evaporation process increases with the following:
1. An increase of a surface area:
If the surface area of the liquid layer is increased, the rate of evaporation increases.
For example, while putting clothes for drying up, we spread them out.
2. An increase of temperature:
When the temperature of the liquid increased, the particles in the liquid gets enough kinetic energy to reach the liquid to vapour state.
3. A decrease in humidity (the amount of water vapour present in air):
When the amount of water in the air significantly high, it decreases the evaporation.
For example, in the cold region, the evaporation process would be slow due to high humidity.
4. An increase in wind speed:
We might have seen that when the wind speed is high, the wet clothes will dry faster. As the wind speed increases, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surrounding.

Evaporation