We have already discussed that,
- Atoms have protons and neutrons in their nuclei.
- Neutrons have no charge, whereas protons have a positive charge.
- The magnitudes of positive and negative charges in an atom are equal, resulting in an electrically neutral atom.
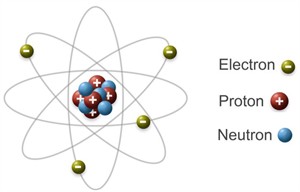
Structure of an atom
Atomic number:
The number of protons in the nucleus of an atom is known as its atomic number. The symbol "\(Z\)" stands for the atomic number. The atom of a different element has a different number of protons.
We can easily calculate the number of electrons or protons in an atom if we know its atomic number.
Let us recall the points for that,
- The number of protons is known as the atomic number.
- In a neutral atom, the number of protons is equal to the number of electrons.
Example:
For carbon, calculate the number of
i. protons and ii. electrons
The atomic number of carbon is \(6\).
We know that the the atomic number is equal to the number of protons.
Hence, the number of protons = \(6\)
In a neutral atom, the number of protons is equal to the number of electrons.
Hence, the number of electrons = \(6\)
Mass number:
The mass number or atomic mass of an atom is equal to the sum of the number of protons and neutrons present in the nucleus. It is represented by the symbol "A"
The atomic number (\(Z\)), mass number (\(A\)), and symbol of an element are written as follows in atomic notation:
Where X is the symbol of an element.
A = Protons + Neutrons
Z = Protons or electrons
Example:
Mass number = \(16\)
Atomic number = \(8\)
By rearranging the atomic mass formula, we can calculate the number of neutrons.
Hence, the number of neutrons = \(16\) - \(8\) = \(8\)
Isotopes:
We observed that some elements have the same atomic numbers but different mass numbers. These are known as isotopes.
Example:
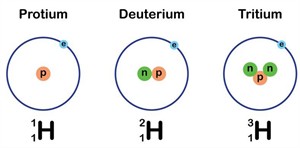
A hydrogen atom with the same atomic number but a different mass number
Most of the elements consist of a mixture of isotopes. They are pure substances. Their chemical properties are similar, but their physical properties are not.
In nature, chlorine exists in two isotopic forms (, ), with masses of \(35\) u and \(37\) u in a \(3\):\(1\) ratio. Obviously, the question that arises here is what mass of chlorine atoms we can use.
Let's see what happens.
The atomic mass of a given element is a weighted average of its isotopes. Each isotope's mass is divided by its abundance.
Application of Isotopes:
- The age of fossils, fuels, and dead organisms is determined using carbon-\(14\) isotopes.
- Cobalt-\(60\) is a radioactive isotope. It decays by emitting gamma rays, which are used to destroy cancer cells.
- In the treatment of goitre, an isotope of iodine is used.
- Blood flow is traced with sodium-\(24\) to identify whether there is an obstruction.
- Uranium-\(235\) is used as fuel in nuclear reactors.
Isobars:
Isobars are atoms with different atomic numbers but the same mass number. In other words, the nucleon count is the same, but the number of protons is different.

Example for isobars
Isobars are different substances, so their chemical properties are different, but their physical properties may be similar because they have the same mass.
Isotones:
Isotones are atoms of different elements with different atomic numbers and mass numbers but the same number of neutrons.
Example: and
Number of neutrons in boron = \(11\) − \(5\) = \(6\)
Number of neutrons in carbon = \(12\) − \(6\) = \(6\)
The above pair of elements, boron and carbon, has the same number of neutrons but different numbers of protons, resulting in different atomic numbers.
oyghhj