J. J. Thomson introduced an atom model, which helped in laying the foundation for further research in the atomic model. Significantly, the research on the atomic model was done by Rutherford.
Rutherford Model:
Ernest Rutherford was curious about the arrangement of electrons in an atom. He designed an experiment in which high-energy alpha particles (\(He^{2+}\)) were used to fall on a thin gold foil. This experiment is also known as the alpha particle scattering experiment.
Observation:
- Since most alpha particles passed through the gold foil without being deflected, most of the space inside the atom is empty.
- Only a few particles were deflected from their direction, suggesting that the atom's positive charge uses very little space.
- Just a small percentage of alpha particles were deflected by \(180°\), showing that the gold atom's positive charge and mass were concentrated in a very small volume within it.
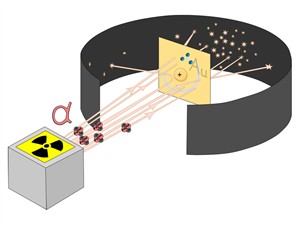
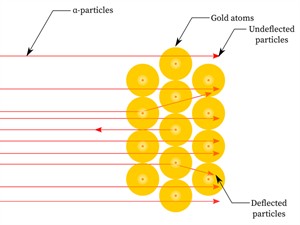
Scattering of \(\alpha\)-particles by a gold foil
Features of the atom:
- The positive center of the atom is known as the nucleus.
- All the mass is concentrated on the nucleus, around which the electrons circulate in the well-defined orbit, much like planets revolving around the Sun.
- The size of the nucleus is less than the atom.
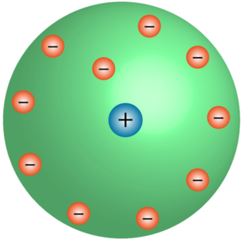
Rutherford's model of an atom
Drawbacks of Rutherford's atomic model:
As electrons revolve in orbit, they accelerate and lose energy. Thereafter, they fall into the nucleus. If this happened, the atom would collapse and matter would no longer exist. Thus, Rutherford's model failed to explain the stability of the atom.
To overcome the drawbacks of Rutherford's model, Neil Bohr proposed a new atomic model.
Postulate of Niel's Bohr's model:
- The electrons revolve around the nucleus in a specific orbit, and these orbits are associated with definite energies called shells or energy levels.
- The electrons do not emit energy when revolving in specific orbits.
These shells, or energy levels, or orbits, are represented by the letters K, L, M, N or by the numbers \(1, 2, 3, 4\).
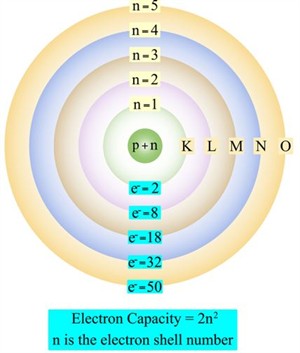
Bohr's atom model (electron shell diagram)
Distribution of electrons in orbits or shells:
Bohr and Bury proposed the distribution of electrons in orbits. The definite distribution of electrons around the nucleus is called electronic configuration.
To achieve the electronic configuration, it follows a certain set of rules:
- The formula \(2n^2\) defines the total number of electrons in a shell. Where n is the energy level or orbit number.
n = \(1,2,3,4,\) etc. Therefore, the maximum number of electrons in different shells are as follows:
| Energy levels | Shells | Maximum electrons | Electron capacity |
|
1
|
K
|
\(2×(1)^2=2\)
|
\(2\)
|
|
2
|
L
|
\(2×(2)^2=8\)
|
\(8\)
|
|
3
|
M
|
\(2×(3)^2=18\)
|
\(18\)
|
|
4
|
N
|
\(2×(4)^2=32\)
|
\(32\)
|
- Unless the inner shells are filled, electrons cannot fill in a given shell. In other words, the shells are gradually filled.
Example:
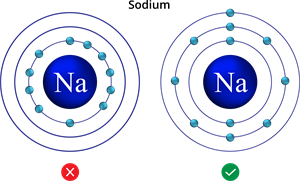
Incorrect and correct filling of electrons in sodium
According to Bohr, the energy of the shell is proportional to its size. The greater the size, the greater the energy. Since the first shell is the smallest, it has the lowest energy, and it is filled first.
Hence, the energy level or size of the shells are given by:
K < L < M < N