In our previous classes, we learnt the fundamental concept of the atom, which is the smallest building block of all matter. Atoms make up everything around us, from the air we breathe to our daily objects. Let’s take a moment to recall what we already know about atoms before exploring their structure in greater detail.
- An atom is the smallest particle.
- Everything around us is made of atoms.
- Atoms are indivisible.
A series of experiments by scientists resulted in the discovery of an atom's structure.
According to Dalton, atoms are indivisible. However, later studies on static electricity and the conditions under which different substances conduct electricity provided the first indications that atoms are not indivisible.
A question arises: Do atoms contain smaller particles inside?
The very question led to many experiments, which eventually revealed the existence of subatomic particles, such as electrons, protons, and neutrons.
According to J.J. Thomson, cathode ray discharge produced new radiations known as cathode rays. These rays carried a negative charge. Cathode rays helped in the discovery of another subatomic particle. The charge of this subatomic particle was the exact opposite of the charge of the proton.
According to E. Goldstein, gas discharge contains new radiations known as canal rays. The rays had a positive charge. Canal rays helped the discovery of another subatomic particle. The charge of this subatomic particle was the exact opposite of the charge of the electron.
Subatomic Particles:
The particles that build an atom are called subatomic particles.
Let's tabulate the names of the scientists who discovered the particles:
|
Subatomic particles
|
Scientists name
|
Symbol
|
Charge
|
Mass (amu) |
|
Electrons
|
J. J. Thomson
|
e−
|
\(−1\)
|
\(0\)
|
|
Protons
|
Eugen Goldstein
|
p+
|
\(+1\)
|
\(1\)
|
|
Neutron
|
James Chadwick
|
\(n^0\)
|
No charge
|
\(1\)
|
Neutrons are present in all atoms except hydrogen.
Atomic mass or mass number = Number of protons + Number of neutrons
As we studied earlier, according to Dalton, atoms are indivisible. However, the discovery of electrons and protons (two fundamental particles) within the atom made Dalton's atomic theory meaningless.
To explain how these particles are arranged inside an atom, J.J. Thomson gave the first model of the atom.
Thomson's model of atom:
J. J. Thomson proposed a model for the atom's structure to explain the arrangement of protons and electrons within the atom. This model is known as Thomson's model of an atom.
Thomson stated that an atom's model resembles that of a Christmas pudding. In a sphere of positive charge, the electrons were like dried fruits in a spherical Christmas pudding.

Thomson's atom (Christmas pudding) model
He also related an atom to a watermelon. The protons are specified by the red component, while the seeds represent the electrons.
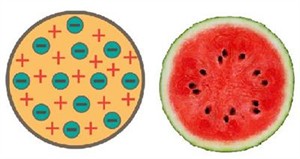
Thomson's atom (Watermelon) model
Proposed Thomson model:
- The electrons are embedded in a positively charged sphere that represents an atom.
- The magnitudes of the negative and positive charges are the same. Hence, the atom is electrically neutral as a whole.
Limitations of J.J. Thomson’s model of the atom:
- It could not explain how positive charge and electrons were arranged inside the atom. It also failed to explain the stability of an atom.
- There was no mention of an atom's nucleus in the theory.
- It was unable to explain Rutherford's scattering experiment.