We have studied how electrons are arranged in an atom across different shells or orbits.
Valence electrons:
The electrons that are found in an atom's outermost orbit are called valence electrons. These electrons determine the chemical reactivity and bonding behavior of an element.
The charge of an atom is decided by the loss or gain of electrons. An atom becomes positive when it loses an electron and negative when it gains an electron.
The difference between valence and charge is that:
-
Valence has no sign and indicates the combining capacity of an atom.
-
Charge has a positive (+) or negative (−) sign and indicates the electrical state of an atom or ion.
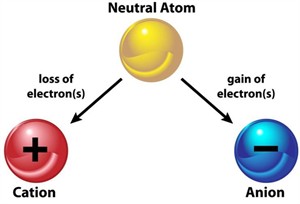
Formation of anion and cation from a neutral atom
Atoms are continually trying to achieve a stable state.
Stable state:
What is meant by a stable state?
If an atom has only one shell, the stable state is achieved when two electrons are present in it. Similarly, when an atom has two shells, the stable state is achieved when the outermost shell has eight electrons.
A few elements such as Helium (He), Neon (Ne), and Argon (Ar) already have this configuration.
This is known as the duplet rule (for one shell) or the octet rule (for more than one shell).
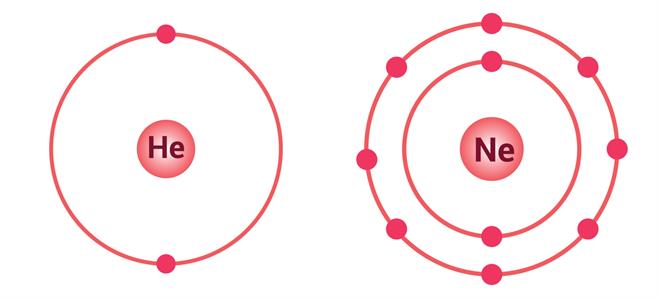
Electron distribution in \(He\) and \(Ne\)
The atoms that do not have this octet are involved in bond formation by gaining, losing, or sharing electrons.
Example for an atom losing an electron: Sodium (\(Na\))
- Atomic number of \(Na\) = \(11\)
- Number of electrons in \(Na\) = \(11\)
- Electronic configuration = \((2, 8, 1)\)
To attain a stable state, sodium loses one electron:
After losing one electron, its configuration becomes (\(2\), \(8\)).
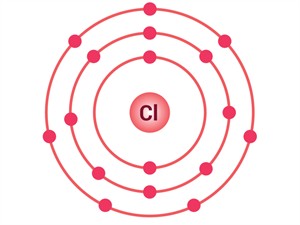
Example for an atom gaining of an electron: Chlorine (\(Cl\))
Atomic number of \(Cl\) = \(17\)
Number of electrons in \(Cl\) = \(17\)
Electronic configuration = \((2, 8, 7)\)
To attain a stable state, chlorine gains one electron:
Who decides how many electrons will be involved in the formation of a bond?

The answer to the above question is "Valency".
Valency:
Valency is the number of electrons an atom can gain, lose, or share to achieve a stable electronic configuration.
The valency of noble gases or inert gases is zero since there are no free electrons in the valence shell, and the elements are already in a stable state.
Example:
Oxygen (O):
- Atomic number = \(8\) → Electronic configuration = \((2, 6)\)
- Needs 2 more electrons to complete its octet.
- Hence, valency = \(2\).
Magnesium (Mg):
Atomic number = \(12\) → Electronic configuration = \((2, 8, 2)\)
Has \(2\) valence electrons; it can either gain \(6\) or lose \(2\).
Losing \(2\) is easier, so valency = \(2\).
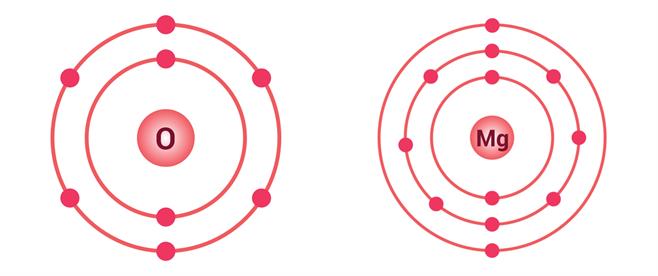
Electron distribution in \(O\) and \(Mg\)
Formation of a bond or compound or molecule:
Let us consider the formation of a sodium chloride (NaCl) molecule.
Sodium (Na) becomes Na⁺, and chlorine (Cl) becomes Cl⁻. The electrostatic attraction between Na⁺ and Cl⁻ forms an ionic bond, resulting in sodium chloride (NaCl).
|
Sodium atom
|
Chlorine atom
|
|
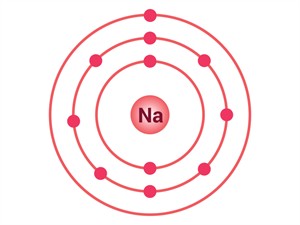
Electrons present = \(11\)
Electronic configuration = (\(2\), \(8\), \(1\))
 |
Electrons present = \(17\)
Electronic configuration = (\(2\), \(8\), \(7\))
 |
Sodium donates one electron to chlorine.
-
Sodium becomes Na⁺ \((2, 8)\)
-
Chlorine becomes Cl⁻ \((2, 8, 8)\)
The electrostatic attraction between Na⁺ and Cl⁻ forms an ionic bond, resulting in the compound sodium chloride (NaCl).
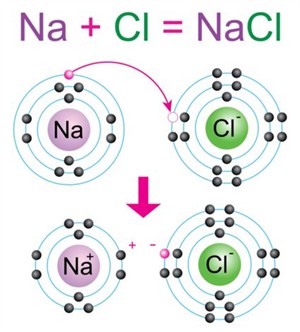
Formation of sodium chloride molecule