We all experience heat in our daily lives. On a sunny day after cycling, you leave the bicycle in the open Sun and rush to the refrigerator to take out ice cubes. When you hold the ice cubes a little longer, it feels cold in your warm hands and it starts melting.

Ice cube melting in warm hands
What’s happening behind the scenes?
- The explanation lies in the way heat moves between objects.
- The heat from your warm hand moves into the colder ice cubes, making your hand feel cold and ice cubes melt by a process called heat transfer.
Heat Transfer:
The movement of heat from a hotter object to a colder object is called heat transfer.
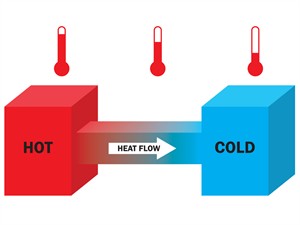
Flow of heat
How is the heat transferred?
Heat is a form of energy that is transferred from one place to another in three different ways.
- Conduction
- Convection
- Radiation
Now you return to the bicycle to start riding again. As soon as you touch the metal handle bar of the bicycle, you feel a sudden burning sensation.

Bicycle parked under the Sun
What’s happening here?
-
The heat from the Sun warms up the metal handle bar of the bicycle.
-
When you touch this hot metal, heat flows from this hotter metal into your cooler hand directly.
-
You do not experience this heat unless you directly touch the metal handle bar.
-
Here the particles in the metal bar do not move but transfer the heat.
-
Since metal is a solid, heat transfer takes place through a process called conduction.
Activity:
To observe how heat is transferred by conduction from a hot region to a cold region without the movement of particles.

Holding a sparkler in hand
Step 1: Take a firework sparkler, light the tip of the sparkler.
Step 2: Hold the other end of the metal rod in your hand.
Step 3: Observe what happens as the sparkler burns.
Step 4: After a while, you will feel the metal rod near your hands getting warmer.
Step 2: Hold the other end of the metal rod in your hand.
Step 3: Observe what happens as the sparkler burns.
Step 4: After a while, you will feel the metal rod near your hands getting warmer.
Explanation:
When the sparkler is lit, heat travels from the burning tip to the cooler end you are holding. This happens through conduction. But the metal particles do not move instead, they only transfer heat to nearby particles, therefore the metal stays in place.
Conduction:
Conduction is a process in which heat is transferred from the hotter part to the colder part of a solid directly without involving any actual movement of the particles of the solid body.
Example:
- At home, we cook food in vessels made of metal. When the vessel is heated, heat is transferred from the metal to the food.
- When we iron clothes, heat is transferred from the iron to the fabric.
If the handlebar is made of wood, will it get as hot as a metal one?
No, because metals conduct heat quickly, they become hot much faster. Wood, on the other hand, does not conduct heat.
That's why metal is called a good conductor of heat, while wood is known as a poor conductor or an insulator.
Good Conductors:
Materials that allow heat to pass through them are called good conductors of heat.
Example:
Steel, iron, aluminium, brass, copper
Bad conductors:
Materials that do not allow heat to pass through them are called bad conductors or insulators of heat.
Example:
Wood, glass, clay, porcelain, plastic, air
Air - A Poor Conductor of Heat:
During winter we all use woollen blankets to keep us warm. Woollen fabric has tiny pores that trap air. As air is a poor conductor of heat, it reduces heat from our body to escape into the surroundings. This helps us feel warm.
Similarly, thermos flasks have a vacuum or a gap of air between their inner and outer layers, which prevents the heat transfer, thus keeping hot drinks hot and cold drinks cold.