Importance:
The chapter "Chemical reactions and equations" carries significant weightage around \(6\) marks, highlighting its significance in the overall curriculum. A clear understanding of this chapter will enhance how substances interact, combine, and transform during various chemical processes.
Question distribution:
- Section A (\(1\) mark) - One question
- Section B (\(2\) mark) - One question
- Section C (\(3\) mark) - One question
(Note: The exact mark distribution may vary slightly across examinations.)
Learning objectives:
- Understanding chemical reactions: Recognise a chemical reaction as a process involving the transformation of reactants into products, indicated by observable changes such as colour, state, temperature, or gas evolution.
- Writing and balancing chemical equations: Learn to represent chemical reactions accurately using symbols and formulas.
- Classify chemical reactions: Identify and differentiate between various types of chemical reactions, such as combination and decomposition.
What is a chemical reaction?
A chemical reaction is a process in which one or more substances (reactants) are converted into new substances (products) with different properties.
Identification of a chemical reaction:
Burning of magnesium ribbon:
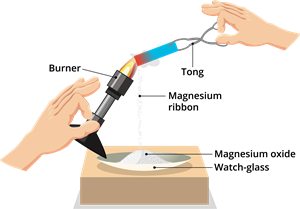
Burning of magnesium ribbon
\(2Mg + O_2 → 2MgO\)
Type of reaction: Combination (also exothermic) reaction.
Exam tip:
- Formation of new substance white ash (\(MgO\))
- Type of reaction - Combination and exothermic (heat is released)
- A dazzling white flame produced
- Metal ribbon (\(Mg\))
Balancing chemical equations:
Example of an unbalanced equation:
\(Fe + H_2O → Fe_3O_4 + H_2\)
Steps to balance (Hit-and-trial method):
- Write a skeletal equation
- Count the atoms of each element on both sides
- Add coefficients to balance
- Check for correctness
- Include physical states
Balanced equation:
\(3Fe({s}) + 4H_2O({g}) → Fe_3O_4({s}) + 4H_2({g})\)
Types of chemical reactions:
Combination reaction:
When two or more reactants combine to form a single product.
\(CaO + H_2O → Ca(OH)_2 + heat\)
Nature: Exothermic (heat is released)
Application (Whitewashing):
\(Ca(OH)_2 + CO_2 → CaCO_3 + H_2O\)
A shiny finish forms on walls due to calcium carbonate (\(CaCO_3\))
Exam tip:
- Exothermic reaction
- Used in whitewashing
- Important compounds: Quick lime (\(CaO\)), slaked lime (\(Ca(OH)_2\)) and limestone (\(CaCO_3\))
- Other examples: Combustion reactions
PYQ - Combination reaction
Decomposition reaction:
A reaction where one reactant breaks down into two or more simpler products.
Note: Most decomposition reactions are endothermic as they require energy to break down compounds. However, the decomposition of organic or vegetable waste into compost is an exothermic process, as it releases heat.
Types of decomposition reaction:
i. Thermal decomposition (by heat):
Example 1:
Hints:
- \(Pb(NO)_3\): White or colourless solid
- \(PbO\): Yellow residue
- \(NO_2\): Brown fumes
Example 2:
Hints:
- Reddish brown residue - \(Fe_2O_3\)
- Pungent smell - \(SO_2\)
Exam tip:
- Balanced chemical equation - Identification of coefficients
- Identification of products and their colour
- Gases evolved
PYQ - Thermal decomposition
ii. Electrolytic decomposition (by electricity):
Example: Electrolysis of water
Exam tip:
- Gas liberated at the cathode (hydrogen) and the anode (oxygen)
- Confirmation test: Hydrogen (pop sound) and oxygen (glows brightly)
- Volume ratio: \(H_2 : O_2\) \(=2:1\)
- A few drops of \(H_2SO_4\) is added to increase conductivity
iii. Photolytic decomposition (by light):
Certain compounds decompose in the presence of sunlight.
Exam tip:
- Pale yellow (\(AgBr_2\)) to greyish white (\(Ag))
- Application: Used in black and white photography
PYQ - Photolytic decomposition
In the next section, we will move ahead to revise displacement reactions, along with the concepts of oxidation, reduction, corrosion, and rancidity, for a complete understanding of this chapter.