Importance:
The chapter "Chemical reactions and equations" carries significant weightage around \(6\) marks, highlighting its significance in the overall curriculum. A clear understanding of this chapter will enhance how substances interact, combine, and transform during various chemical processes.
Question distribution:
- Section A (\(1\) mark) - One question
- Section B (\(2\) mark) - One question
- Section C (\(3\) mark) - One question
(Note: The exact mark distribution may vary slightly across examinations.)
Learning objectives:
- Classify chemical reactions: Identify and differentiate between various types of chemical reactions, such as displacement reaction.
-
Apply oxidation and reduction concepts: Understand oxidation and reduction processes in reactions and recognise their importance in real-life phenomena like corrosion.
-
Relate chemical reactions to daily life: Relate concepts like corrosion and rancidity to real-life scenarios, understanding their causes and preventive measures and their impact on materials and food.
Displacement reaction:
A more reactive element displaces a less reactive element from its compound. It is also called as single displacement reaction.
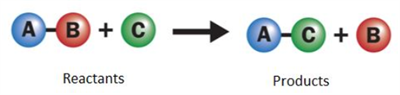
Single displacement reaction
Example:
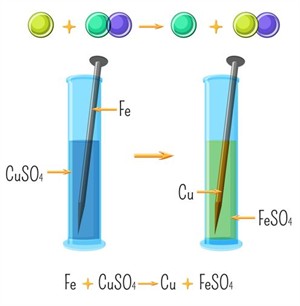
Reaction between iron and copper sulphate
Hints:
- Blue colour of copper sulphate solution fades and a pale green colour iron sulphate solution is formed.
- Brown coating forms on the nail.
- Iron is more reactive and displaces copper.
Exam tip:
- Recall the reactivity series
- Observe colour change as a key indicator
Double displacement reaction:
The exchange of ions between the reactants to form new products. Double displacement reaction has the following two types:
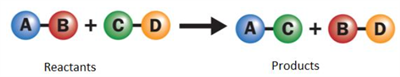
PYQ - Displacement reaction
i. Neutralisation reaction:

Hint:
Acid + Base → Salt + Water
Exam tip:
- Type of reaction - Double displacement
- Example - Equation
PYQ - Neutralisation reaction
ii. Precipitation reaction:
Hints:
1.
Reactants: Both are colourless solutions
Products: White precipitate (\(BaSO_4\))
2. \(Pb(NO_3)_2(aq) + 2KI(aq) → PbI_2(s) + 2KNO_3(aq)\)
Reactants: Both are colourless solutions
Products: Yellow precipitate \(PbI_2\)
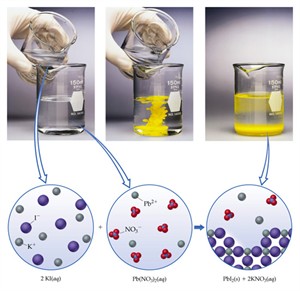
Precipitation of lead iodide
Exam tip:
- Type of reaction - Double displacement
- Find the products
- Insoluble substance formed
- Include physical states (solid) and downward arrow
- Balanced chemical equation
Redox reaction:
| Oxidation | Reduction |
| Gain of oxygen | Loss of oxygen |
| Loss of hydrogen | Gain of hydrogen |
| Loss of electron | Gain of electron |
| Increase in oxidation number | Decrease in oxidation number |
Redox: Both oxidation and reduction occur simultaneously.
Example:

Redox reaction
Hints:
- Heat copper powder → turns black (\(CuO\))
- Pass hydrogen → turns brown again (\(Cu\) metal)
Exam tip:
- Copper oxide gets reduced
- Hydrogen gets oxidised
| Agent | What is does? | What happens to it? | Example |
| Oxidising agent | Causes oxidation of other substance | It gets reduced |
In \(CuO + H_2 → Cu + H_2O\),
\(CuO\) is the oxidising agent
|
| Reducing agent | Causes reduction of other substance | It gets oxidised |
In \(CuO + H_2 → Cu + H_2O\),
\(H_2\) is the reducing agent
|
Corrosion:
Corrosion is the progressive destruction of metals by the action of air, moisture or chemicals (such as an acid) on their surface.
Hints:
- Iron oxide - Reddish brown (Rust)
- Silver sulphide - Black (Tarnish)
- Copper carbonate - Green (Patina)
Exam tip:
- Prevention
- Colour change
- Condition: Rust - Oxygen (air) + Moisture (water)
Rancidity:
The oxidation of oils and fats present in food material resulting in bad smell and taste.
Hints:
Methods to prevent rancidity - Antioxidants, air tight containers, and flushing packets with nitrogen.
Exam tip:
- Prevention: Chips packet flushed with nitrogen gas - Inert
- Oxidation
PYQ - Rancidity
Change in temperature:
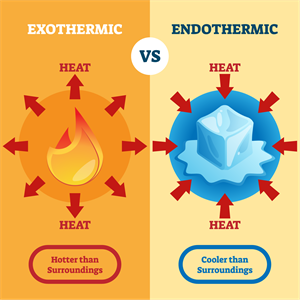
Exothermic vs Endothermic
Hints:
- Exothermic - Release of heat
- Endothermic - Absorption of heat
Exam tip:
- Endothermic examples: Photosynthesis, melting of ice, cooking of egg, decomposition (mostly), dissolving ammonium chloride/nitrate, sublimation.
- Exothermic examples: Combustion, combination, respiration, rusting of iron, neutralisation (mostly), decomposition of vegetable waste.